More Information
Submitted: September 06, 2022 | Approved: September 15, 2022 | Published: September 16, 2022
How to cite this article: Uppal R, Uppal MS, Khan AA, Saeed U. SARS-CoV-2 Omicron and centaurus variants induced lymphocytopenia: A multicenter clinical investigation on 118,561 cases across Pakistan during 2021-2022. Int J Clin Virol. 2022; 6: 034-037.
DOI: 10.29328/journal.ijcv.1001047
Copyright License: © 2022 Uppal R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: SARS-CoV-2; Omicron; Centaurus; Lymphopenia; Biomarkers; Pakistan
SARS-CoV-2 Omicron and centaurus variants induced lymphocytopenia: A multicenter clinical investigation on 118,561 cases across Pakistan during 2021-2022
Rizwan Uppal1, Muhammad Saad Uppal1, Aftab Ahmad Khan1 and Umar Saeed1,2*
1Department of Research and Development, Islamabad Diagnostic Center (IDC), F8 Markaz, Islamabad (44000), Pakistan
2Internatioanl Center of Medical Sciences Research (ICMSR), Islamabad (44000), Pakistan
*Address for Correspondence: Umar Saeed, Department of Research and Development, Islamabad Diagnostic Center (IDC), F8 Markaz Islamabad (44000), Pakistan, Email: [email protected]
The SARS-CoV-2 pandemic is still ongoing. Previously, several studies have been conducted to investigate laboratory markers as a tool for severity assessment during COVID-19 infections. Biological markers such as Platelet count, D-dimer and IL-6, Lymphocytopenia and others have been used for assessment of severity in COVID-19 disease patients (infected by SARS-CoV-2 Alpha, Beta, Gamma, Delta, Epsilon, and other variants). We observed a significant drop in lymphocyte count among suspected SARS-CoV-2 clinical patients with symptoms of fever, running nose, breathing discomfort, cough, and others during Omicron and Centaurus variants spread in Pakistan. A multicenter, cross-sectional study was conducted from Jan 2021 to Aug 2022, on 118,561 subjects to evaluate hematological abnormalities among suspected patients. Of note, significantly decreased lymphocyte levels (lymphocytopenia) were observed among 43.05% of infected patients. Also, the levels of NA (39.03%), HGB (28.27%), MCV (22.62%), PLT (8.17%), and ALB (4.30%) were also reduced among infected patients. This suggests that lymphopenia can be used as an alternative, cost-effective, early diagnostic biomarker for clinical COVID-19 patients, even before the diagnosis via real-time PCR. In resource-limited countries, the current study is critical for policy-making strategic organizations for prioritizing lymphocytopenia-based screening (as an alternative, cost-effective diagnostic test) in clinical COVID-19 patients, before real-time PCR-based diagnosis.
SARS-CoV-2 has been declared a global public health emergency by World Health Organization (WHO) due to its pandemic nature [1,2]. Since the passage of 2.5 years (since Dec 2019), the global number of SARS-CoV-2 cases is increasing day by day. Despite the availability of COVID-19 vaccines, due to the limited supply chain across the globe and rapidly evolving variants of SARS-CoV-2, the war against COVID-19 is still not over. To date, 605,189,782 cases of SARS-CoV-2 have been reported worldwide [3].
SARS-CoV-2 patients infected with Omicron and Centaurus variants suffer from fever, body aches, cough, and flu-like symptoms; less likely may develop severe pneumonia, respiratory discomfort, difficulty in breathing, acute respiratory distress syndrome, or multi-organ failure, and death [4]. In Pakistan, the SARS-CoV-2-related infection surpassed 1,568,183 cases. Despite successful vaccination (among 130,985,253 individuals) against SARS-CoV-2, due to rapidly evolving SARS-CoV-2 variants and immune evasion, the available vaccines could not fully protect against COVID-19 [5]. Among SARS-CoV-2 deceased patients, a significantly lower count of lymphocytes was observed in China. Multiple studies have been conducted to analyze biomarkers including lymphocyte count, for determining the severity of disease in SARS-CoV-2 patients [5].
Here we conducted analysis during Omicron and Centaurus variants spread in Pakistan and enrolled suspected SARS-CoV-2 patients for the evaluation of complete blood profile; including mean corpuscular hemoglobin (MCH), neutrophils (NEU), lymphocytes (LYMP), hematocrit test (HCT), Absolute Monocyte Count (AMC), absolute eosinophil count (EOS), mean corpuscular hemoglobin concentration (MCHC), monocytes count (MONO), hemoglobin count (HGB), red blood cells (RBC), platelet count (PLT), white blood cell count (WBC), Red Cell Distribution Width (RDW), eosinophils (EOS), Absolute Neutrophil Count (ANC), Absolute Lymphocyte Count (ALC), Mean Corpuscular Volume (MCV), Creatinine (CR), Albumin (ALB), Sodium Blood test (NA), Ferritin (FER), C-Reactive Protein (CRP), D-dimer (DD) tests.
A cross-sectional study was conducted at Islamabad Diagnostic Center, having more than 100 branches across Pakistan, on COVID-19 patients from January 2021- August 2022; to examine complete blood profiles among suspected SARS-CoV-2 subjects. Pre-test counseling was performed by trained phlebotomists and counselors. All enrolled participants were informed prior to the examination and the patient’s consent was obtained. The nasopharyngeal swab samples and blood specimens were obtained from all suspected clinical COVID-19 Omicron and Centaurus patients having fever and/or respiratory symptoms.
The nasopharyngeal swab samples were examined for SARS-CoV-2 via real-time PCR after RNA extraction by using Auto pure 32 Zybio, China. As per recommendations of WHO, USFDA approved triple target gene (SARS-CoV-2 RNA dependent RNA polymerase gene, N gene, and Sarbecovirus E gene) based detection Allplex 2019-nCoV Assay (Seegene, South Korea). Cycle threshold values < 40 were considered SARS-CoV-2 positive. Potential hematological alterations were examined for LYMP, MCH, NEU, HCT, AMC, EOS, MCHC, MONO, HGB, RBC, PLT, WBC, RDW-CV, EOS, ANC, ALC, MCV, CR, ALB, NA, FER, CRP, DD tests, using Sysmex XN-9000 automated analyzer (Sysmex, Kobe, Japan) based on flow cytometer that utilized impedance principle linked to the optical system via laser emitted beam of light.
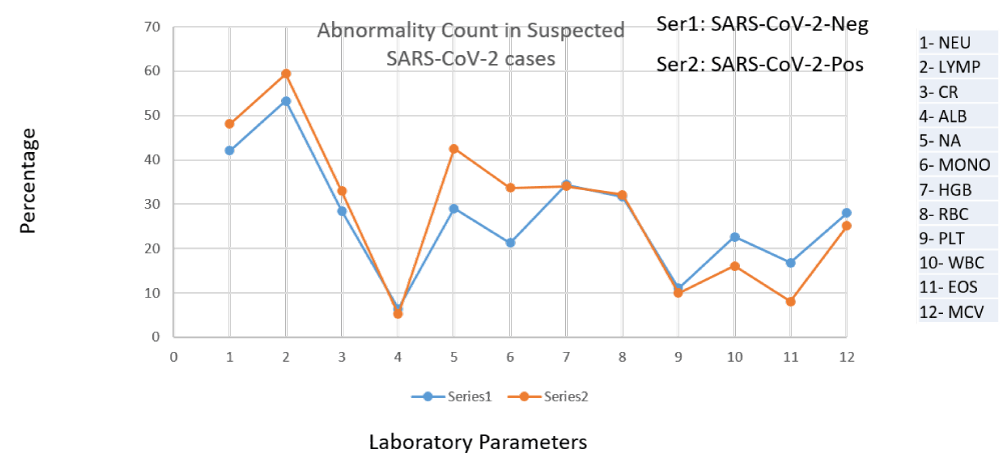
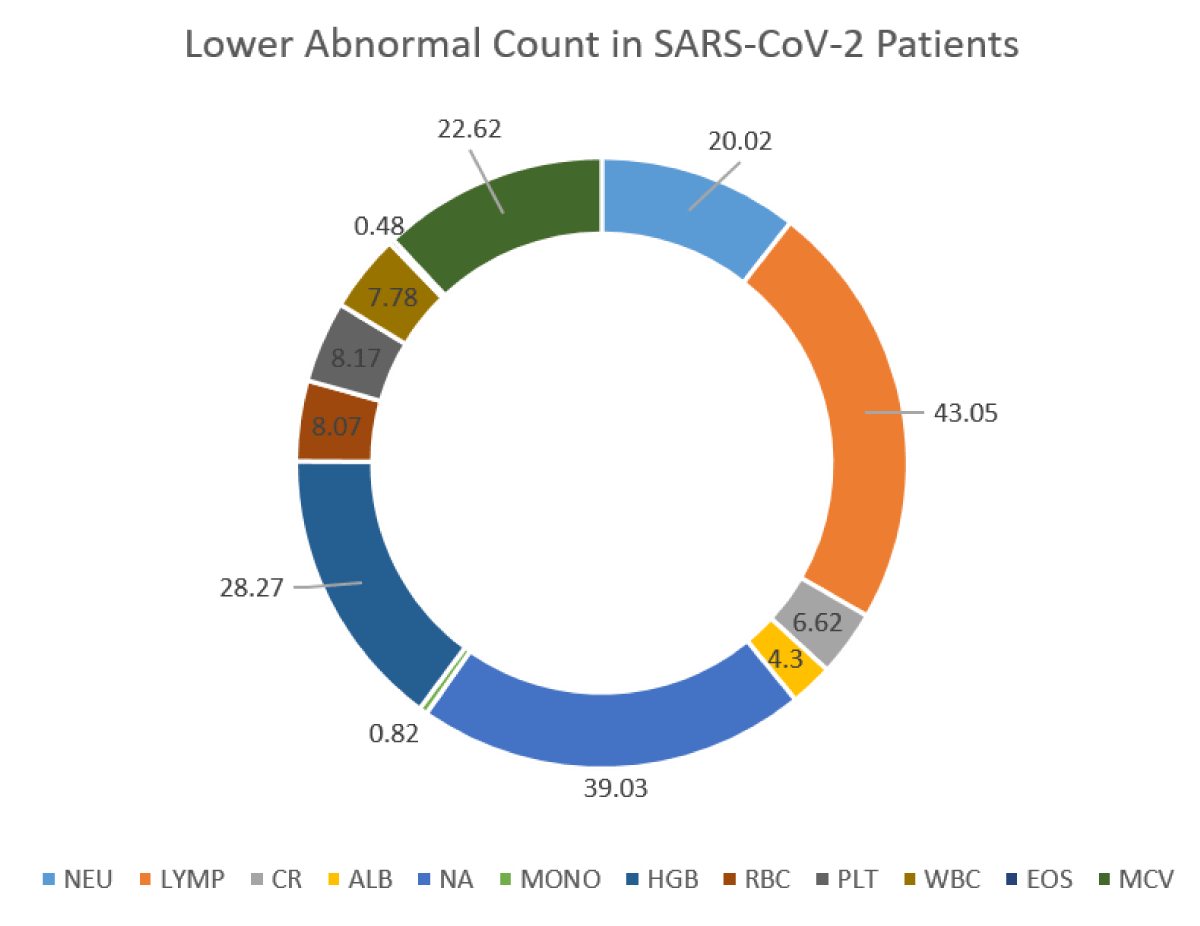
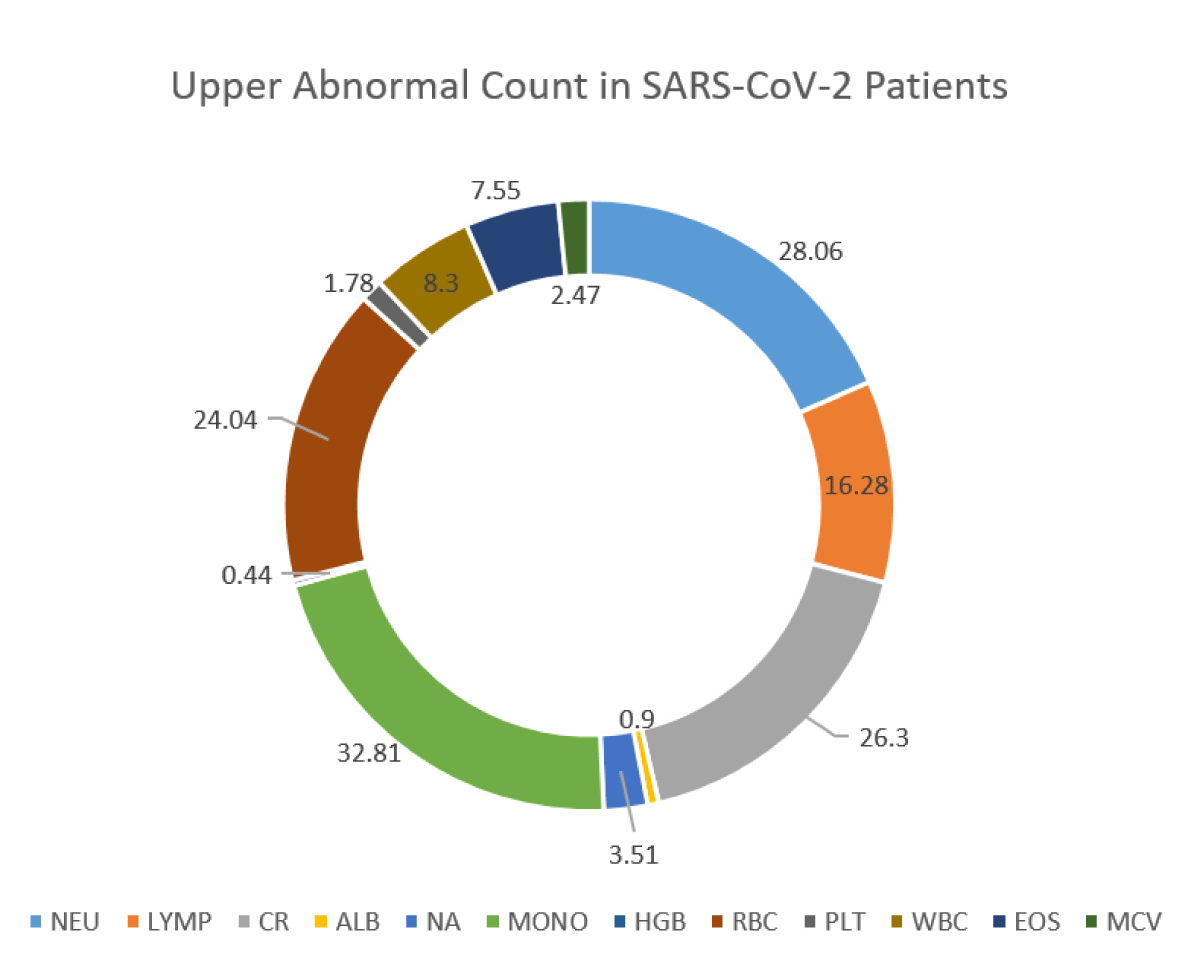
118,561 suspected Omicron and Centaurus SARS-CoV-2 variants were enrolled for the study. 41,698 individuals were confirmed SARS-CoV-2 positive (detected via real-time PCR), and 76,863 individuals were found negative. Among Omicron and Centaurus variants confirmed SARS-CoV-2 positive patients, 55.17% were males and 44.83% were females. In comparison to SARS-CoV-2 negative participants with altered hematological profiles (27.01%), the SARS-CoV-2 Centaurus and Omicron positive patients depicted relatively more hematological abnormalities (28.42%) as shown in Figure 1. Of note, among SARS-CoV-2 infected individuals, drastically reduced levels of LYMP (43.05%) were observed, indicating the significant potential of lymphocyte count as an alternative clinical diagnostic parameter for clinical COVID-19 patients. Furthermore, NA (39.03%), HGB (28.27%), MCV (22.62%) PLT (8.17%), and ALB (4.30%) levels were reduced, as shown in Figure 2. Abnormally raised levels of NEU, CR, MONO, RBCs, WBC, and EOS were found among 28.06%, 26.3%, 32.81%, 24.04%, 8.3%, and 7.55% subjects respectively as shown by Figure 2.
Figure 1: Abnormal complete blood parameters among suspected SARS-CoV-2 patients.
Figure 2a: Abnormality of blood parameters among SARS-CoV-2 patients.
Figure 2b: Abnormality of blood parameters among SARS-CoV-2 patients.
Among SARS-CoV-2 positive patients, in contrast to SARS-CoV-2 negative patients abnormal blood parameters count included LYMP count among 59.41% (in comparison to 53.3%), abnormal NEU count were found among 48.1% (in comparison to 42.1%), abnormal EOS count among 64.5% (compared to 39.9% of SARS-CoV-2 Neg), abnormal MONO count in 33.62% (than 21.32% of SARS-CoV-2 Neg), abnormal RBC count in 32.33% (than 31.74%), abnormal ALC count among 45.62% (than 31.88%), abnormal CR count among 32.92% (than 28.4%), abnormal NA count among 42.54% (than 29%), abnormal CRP count among 70.47% (than 63.80%), abnormal DD among 65.09% (than 59.24%) individuals.
The severity of SARS-CoV-2 infected individuals has been determined by several biological markers including lymphopenia, Platelet count, D-dimer and IL-6, and others, as evaluated by similar type of studies conducted on beta-CoV infection, SARS-CoV, and the Middle East respiratory syndrome (MERS-CoV) [6,7]. CD4+ and CD8+ lymphocytes may drastically fall during SARS-CoV infection possibly due to lymphocyte sequestration at distinct target organ sites [7,8]. SARS-CoV structurally resembles MERS-CoV, both bind to different receptors during entrance to the cell. For entrance to the cell; the SARS-CoV binds to angiotensin-converting enzyme 2 (ACE2), however, MERS-CoV binds to cellular dipeptidyl peptidase 4 (DPP4) [9,10]. Viral infections are increasing day by day and there is a dire need for reliable viral diagnostic methods to prevent the future burden of diseases [11-21].
The mechanism of lymphocyte reduction during SARS-CoV-2 has been poorly understood. Besides lymphocyte infiltration and pulmonary sequestration or sequestration in lymphoid tissues, it might be possible that ACE2 receptor expressing lymphocytes may facilitate SARS-CoV-2 infection to the lymphocytes, and subsequent release of Interleukin 6 (a pro-inflammatory cytokine) may downregulate lymphocyte levels [7,10]. Herein we found a significant decrease in lymphocyte count among suspected clinical SARS-CoV-2 patients with symptoms of fever, cough, running nose, breathing discomfort, flu-like symptoms, and others.
Inconsistent with our previous findings on suspected SARS-CoV-2 patients, during the first three waves of COVID-19 [Alpha variant (B.1.1.7), delta variant, epsilon variant, and other variants) in Pakistan, where several biomarkers (CRP, DD, FER, hemoglobin A1c (HBA1c), interleukin 6 (IL6), lactate dehydrogenase (LDH), NT-pro-B-type natriuretic peptide (PBNP), and procalcitonin (PCT) and correlated high-resolution tomography results were evaluated; during the current study with prevalent Omicron variant of SARS-CoV-2, also depicted significantly raised levels of FER (47.67%) and CRP (70.47%) [4].
Lymphocytopenia, in addition to a few other diagnostic parameters, is a known biomarker to access the severity of COVID-19. Herein we first time demonstrated that lymphocytopenia can be used as a cost-effective diagnostic biomarker among clinical Omicron and Centaurus SARS-CoV-2 patients with symptoms of fever, body aches, cough, respiratory discomfort, flu-like symptoms, and others, prior to real-time PCR based testing.
We would like to acknowledge Assistant Manager Database, Mr. Muhammad Rizwan from Islamabad Diagnostic Center (IDC), for his kind support in data management.
Authors contribution
RU is the principal investigator (Co-PI) of the study. RU and US conceived the study. MSU conducted the research analysis. AAK assisted the US in approval of the study. The US wrote the manuscript and is Co-PI of the study.
- World Health Organization. Coronavirus disease (COVID-19) outbreak 2022. https://www.who.int/westernpacific/emergencies/covid-19.
- World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 79. World Heal. Organ. 2020.
- www.woldometers.info/coronavirus/
- Saeed U, Uppal SR, Piracha ZZ, Rasheed A, Aftab Z, Zaheer H, Uppal R. Evaluation of SARS-CoV-2 antigen-based rapid diagnostic kits in Pakistan: formulation of COVID-19 national testing strategy. Virol J. 2021 Feb 13;18(1):34. doi: 10.1186/s12985-021-01505-3. PMID: 33581714; PMCID: PMC7881305. https://doi.org/10.1186/s12985-021-01505-3.
- https://covid.gov.pk/
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 May;46(5):846-848. doi: 10.1007/s00134-020-05991-x. Epub 2020 Mar 3. Erratum in: Intensive Care Med. 2020 Apr 6;: PMID: 32125452; PMCID: PMC7080116.
- Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020 Dec;9(1):727-732. doi: 10.1080/22221751.2020.1746199. PMID: 32196410; PMCID: PMC7170333.
- Li T, Qiu Z, Zhang L, Han Y, He W, Liu Z, Ma X, Fan H, Lu W, Xie J, Wang H, Deng G, Wang A. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. 2004 Feb 15;189(4):648-51. doi: 10.1086/381535. Epub 2004 Feb 4. PMID: 14767818; PMCID: PMC7109946.
- Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013 Mar 14;495(7440):251-4. doi: 10.1038/nature12005. PMID: 23486063; PMCID: PMC7095326.
- Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020 Feb 24;12(1):8. doi: 10.1038/s41368-020-0074-x. PMID: 32094336; PMCID: PMC7039956.
- Saeed U, Uppal SR, Piracha ZZ, Uppal R. COVID-19 Transmission via Fomites at Low Temperature: A Potential Silent SARS-CoV-2 Propagation Route. Am J Biomed Sci & Res. 2021 - 12(1)? AJBSR.MS.ID.001716.
- Saeed U, Uppal SR, Piracha ZZ, Khan AA, Rasheed A, Zaheer H, et al. Effectivity analysis of SARS-CoV-2 nasopharyngeal swab rapid testing kits in Pakistan: A scenario of inadequate COVID-19 diagnosis. Research Square. 2021; Doi: 10.21203/rs.3.rs-315851/v1.
- Saeed U, Manzoor S. Risk factors associated with transmission of hepatitis B and hepatitis C virus in Pakistan. Glob J Med Res. 2014; 14(1):14–9.
- Saeed U, Waheed Y, Ashraf M. Hepatitis B and hepatitis C viruses: a review of viral genomes, viral induced host immune responses, genotypic distributions and worldwide epidemiology. Asian Pac J Trop Dis. 2014; 4(2):88–96. Doi: 10.1016/s2222-1808(14)60322-4.
- Saeed U, Waheed Y, Ashraf M, Waheed U, Anjum S, Afzal MS. Estimation of hepatitis B virus, hepatitis C virus, and different clinical parameters in the thalassemia population of capital twin cities of Pakistan. Virology (Auckl). 2015; 6:11–6. Doi: 10.4137/VRT.S31744.
- Saeed U, Rizwan Uppal S, Zahid Piracha Z, Uppal R. Azithromycin treatment for SARS-CoV-2-related COVID-19 pandemic could worsen extensively drug resistant XDR typhoid: A risk of losing the last bullet against Salmonella enteric serovar typhi. Jundishapur J Microbiol. 2021; 14(1). e113874. Doi: 10.5812/jjm.113874.
- Saeed U, Uppal SR, Piracha ZZ, Uppal R. COVID-19 transmission via fomites at low temperature: A potential silent SARS-CoV-2 propagation route. Am J Biomed Sci & Res. 2021; 12(1):80–2.
- Saeed U, Uppal SR, Piracha ZZ, Uppal R. SARS-CoV-2 Spike Antibody Levels Trend among Sinopharm Vaccinated People. Iran J Public Health. 2021; Jul50(7):1486-1487. doi: 10.18502/ijph.v50i7.6640. PMID: 34568189; PMCID: PMC8426791.
- Saeed U, Uppal SR, Piracha ZZ, Khan AA, Rasheed A, Waheed A. Evaluation of SARS-CoV-2 spike antibody levels among Sputnik V first dose vaccinated people in Pakistan: formulation of national anti COVID-19 mass vaccination strategy. Res Sq. 2021; Doi: 10.21203/rs.3.rs480406/v1.
- Saeed U, Piracha ZZ, Uppal R, Uppal R. SARS-CoV-2-Associated CRP, DD, FER, HBA1c, IL6, LDH, PBNP, and PCT Biomarkers and High-Resolution Computed Tomography During the First Three Waves of COVID-19 in Pakistan 2019-2021. Jundishapur J Microbiol. 2022; 15(1):e119590.
- Uppal SR, Khan AA, Saeed U, Piracha ZZ. Dermatomycoses in Pakistan; an urgent need for National Surveillance Programs. Ann Dermatol Res. 2022; 6: 014-016.