More Information
Submitted: February 06, 2021 | Approved: February 15, 2021 | Published: February 16, 2021
How to cite this article: Luisetto M, Almukthar N, Hamid GA, Tarro G, Edbey K, et al. COVID-19 New variant and air pollution relationship: how airborne mutagens agent can act on genoma viruses expression: Hypothesis of work. Int J Clin Virol. 2021; 5: 022-031.
DOI: 10.29328/journal.ijcv.1001031
Copyright License: © 2021 Luisetto M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Air pollution; Environmental toxicology; COVID-19 new variant; Mutagen; Particulate matter; NO2; Benzo-a-pyrene; Cause-effect relationship; Direct or indirect actions
COVID-19 New variant and air pollution relationship: how airborne mutagens agent can act on genoma viruses expression: Hypothesis of work
Luisetto M1*, Naseer Almukthar2, Gamal Abdul Hamid3, Tarro G4, Khaled Edbey5, Nili BA6, Ghulam Rasool Mashori7, Ahmed Yesvi Rafa8 and Latyshev O Yu9
1IMA Marijnskaya Academy, Environmental Toxicology, Italy
2Professor, Department of Physiology/College of Medicine University of Babylon, Iraq
3Professor, Hematology Oncology, University of Aden, Aden, Yemen
4President of the T & L de Beaumont Bonelli Foundation for Cancer Research, Naples Italy
5Professor, Department of Chemistry, University of Benghazi, Libya
6Innovative Pharmaceutical Product Development Specialist, USA
7Full Professor of Pharmacology, Peoples University of Medical and Health Sciences for Women, Nawabshah, Pakistan
8Founder and President, Yugen Research Organization, Western Michigan University, MI, USA
9IMA Academy, President, Russian Federation
*Address for Correspondence: Luisetto M, IMA Marijnskaya Academy, Environmental Toxicology, 29121 Italy, Te: +393402479620; Email: [email protected]
Before actual COVID-19 pandemia coronavirus was not so dangerous like now.
In December 2019 - January 2020 in Wuhan first and then in other places this coronavirus was responsible of a first wave of severe pulmonitis responsible of many deaths.
Wuhan and other region involved first was high level air polluted and industrial area.
New COVID-19 variant in last part of 2020 and in first month of 2021 was responsible of great diffusion of this pandemic disease.
UK, South Africa and brasilian new variant show higher diffusion then the first wave of COVID-19.
Aim of this work is to analyze relationship with air pollution and the possibility that mutagen substantia inside of this microenvironment can produce new variant trough an genetic pressure process.
RNA viruses are normally subjected by natural mutation but some phenomena can contribute to accelerate this process and their airborne – aeresols microenvironment is relevant.
Some air pollutants are recognized as mutagen factors by literature.
Coronaviruses are a group of related RNA- viruses that cause diseases in mammals and birds. They cause respiratory- tract infections that can range from mild to lethal.
Mild illnesses in humans include some cases of the common cold (which is also caused by other viruses, predominantly like the rhinoviruses), while more lethal -varieties can cause SARS, MERS, and COVID-19 disease.
The first reports of a coronavirus- infection in animals occurred in the late 1920-1930s, when an acute respiratory infection of domesticated chickens emerged in the North America.
The virus was later known as an infectious bronchitis- virus (IBV).
The most recent common ancestor of all coronaviruses is estimated to have existed as recently as about 8000 before common era, and some models back about 50 million years, this imply a long term coevolution with bat and avian species.
Bats and birds, as warm-blooded flying vertebrates, are considered natural reservoir for the coronavirus gene pool.
In last 2020 and in first month of 2021 new COVID-19 VARIANT was responsible of great spread of the disease more higher then in the first wave.
Because relevant implication in public health politics it is really crucial to investigate some factor that can be considered to explain this phenomena.
If RNA virus are involved in various kind of natural mutation process the relationship with some Polluted area and new variant explosion is interesting fact to be observed.
South UK, Manaus – Brazil amazon zone and south Africa show a characteristic air pollution condition and the same it is interesting to observe that new variant emerged. (see researche article Deforestation, air pollution and Brasiliant COVID-19 variants Luisetto, et al. Jan 2021. Preprint Researchgate).
Form CDC website New Variants of the Virus that Causes COVID-190
Updated Jan. 28, 2021
“Multiple variants of the virus that causes COVID-19 are circulating globally:
The United Kingdom (UK) identified a variant named B.1.1.7 with a large number of mutations in the fall of 2020. This variant spreads more easily and quickly than other variants. In Jan. 2021, experts and researcher in the UK reported that this variant may be associated with an increased risk level of death compared to other variant viruses, but more research studies are needed to confirm this finding. It has since been detected in many countries around the world. This variant was first detected in the US at the end of Dec. 2020.
In South Africa S.A., another variant called B.1.351 emerged (Independently of the B.1.1.7). Originally detected in early October 2020, B.1.351 shares some mutations with B.1.1.7. Cases caused by this variant have been reported in the US at the end of Jan- 2021.
In Brazil, a variant called P.1 emerged that was first identified in travelers from Brazil country, who were tested during routine screening at an airport in Japan, in early Jan. This variant contains a set of additional mutations that may affect its ability to be recognized by antibodies. This variant was first detected in the US at the end of January 2021”
And from WHO Coronavirus disease (COVID-19): Virus Evolution Dec. 2020.
“When a virus replicates or makes copies of itself, it sometimes changes a little bit. These changes are named “mutations.” A virus with one or several new mutations is referred to as a “variant” of the original virus.
So the more viruses circulate, the more they may change. These changes can occasionally result in a virus variant that is better adapted to its environment compared to the original- virus. This process of changing and selection of successful variants is named “virus evolution.”
Some mutations can lead to changes in a virus’s characteristics, such as altered transmission (it may spread more easily) or severity (causing more severe- disease).
Related velocity: Some viruses change quickly and others more slowly.
SARS-CoV-2 tends to change more slowly than others such as HIV or influenza viruses. This could in part be explained by the virus’s internal “proofreading- mechanism” which can correct the “mistakes” when it makes copies of itself. Scientists -and researcher continue to study this mechanism to better understand how it works.”
Genetic Variants of SARS-CoV-2—What Do They Mean?
Adam S. Lauring, et al: JAMA. Published online January 6, 2021. doi:10.1001/jama.2020.27124.
“Over the course of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, the scientific, and public health institutions have had to respond to new viral genetic- variants. Each one has triggered a flurry of the media attention, a range of reactions from the scientific community, and calls from governments to either “stay calm” or pursue immediate- countermeasures. While many scientists were initially skeptical about the significance of the D614G alteration, the emergence of the new “UK variant”—named lineage B.1.1.7—has raised widespread concern. Understanding which variants are concerning, and why, requires an appreciation of virus- evolution and the genomic epidemiology of SARS-CoV-2. Mutations arise as a natural by-product of viral -replication. RNA- viruses typically have an higher mutation rates than the DNA viruses. Coronaviruses, make fewer mutations than most RNA- viruses because they encode an enzyme that corrects some of the errors made during replication. In most cases, the fate of a newly arising mutation is determined by natural -selection. Those that confer a competitive- advantage with respect to viral replication, transmission, or escape from immunity will increase in frequency, and those that reduce viral fitness tend to be culled from the population of circulating- viruses. But mutations can also increase and decrease in frequency due to chance events.
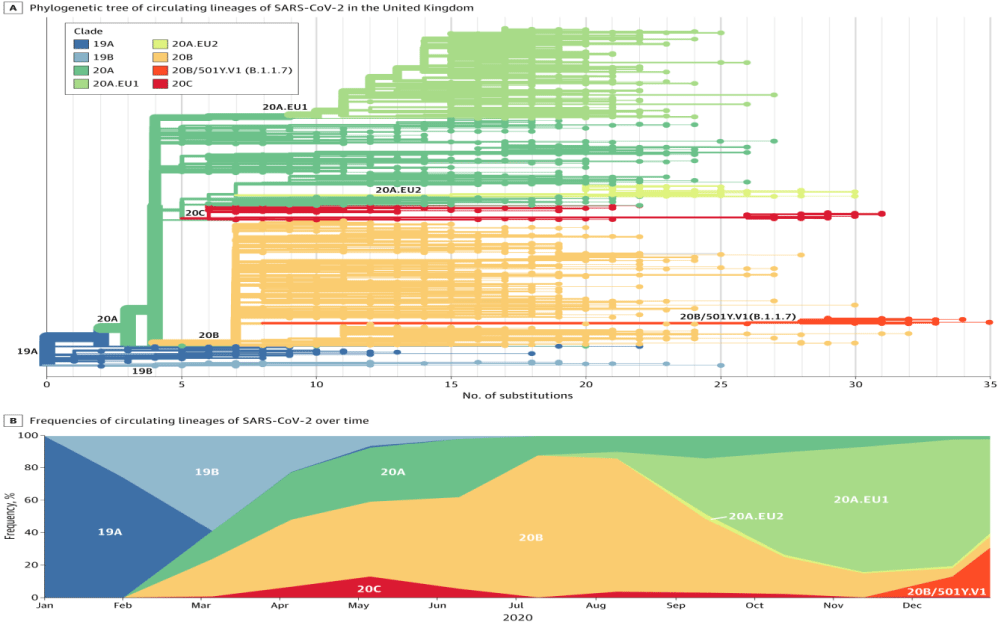
In example, a “founder effect” occurs when a limited number of individual viruses establish a new population during the transmission. The mutations present in the genomes of these viral- ancestors will dominate the population regardless of their effects on viral fitness. This same interplay of natural- selection and chance events shapes virus evolution within hosts, in communities, and across countries. Although the terms mutation, variant, and strain are often used inter-changeably in describing the epidemiology of SARS-CoV-2, the distinctions are important. Mutation refers to the actual change in sequence: D614G is an aspartic acid-to-glycine substitution at position 614 of the spike- glycoprotein SPG. Genomes that differ in sequence are often named variants. This term is somewhat less precise because 2 variants can differ by 1 mutation or many. Strictly speaking, a variant is a strain when it has a demonstrably different phenotype (a difference in antigenicity, transmissibility, or virulence)” (Figures 1,2 ).
Figure 1: A - Phylogenetic tree: showing the relationship of lineage B.1.1.7 (20B/501Y.V1, orange branch and tips) to other circulating- lineages. The long branch length for this lineage reflects the fact that it accumulated a significant number of mutations prior to being discovered. B, Frequencies of circulating lineages over the time. Lineages are colored as in the tree, with lineage B.1.1.7 (20B/501Y.V1) shown in orange (From JAMA).
Figure 2: A new SARS-CoV-2 variant referred to as SARS-CoV-2 VUI 202012/01 (Variant Under Investigation, year 2020, month 12, variant 01), has been declared by the British -authorities. The variant was detected via genomic- sequencing in the UK. It is defined by multiple spike protein mutations (deletion 69-70, deletion 144, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H) present.
The new -variant is correlated with a rapid increase in the COVID-19 incidence. This increase was reported in South- East England. Analysis using viral genome -sequence data identified a large proportion (> 49% - 50%) of cases that belonged to a new single lineage. Overall, around 5% to 10% of all COVID-19 cases are regularly sequenced in the UK. As of 26 Dec. 2020, about more than 3000 individuals had been identified with this virus variant in England, with the earliest case identified from 20 Sep. 2020. Several countries had reported sporadic- cases caused by the new variant from other EU/EEA countries (Belgium, Denmark, France, Germany, Iceland, Ireland, Italy, Netherlands, Norway, Portugal, Spain and Sweden) and globally (Australia, Canada, Hong -Kong, Israel, Japan, Jordan, Lebanon, South Korea, Switzerland, Singapore).
South Africa S.A reported another SARS-CoV-2 variant of potential concern, designated 501.V2 that was first observed in samples from Oct. and since then evolved to be the dominant circulating variant in South Africa (From LNS).
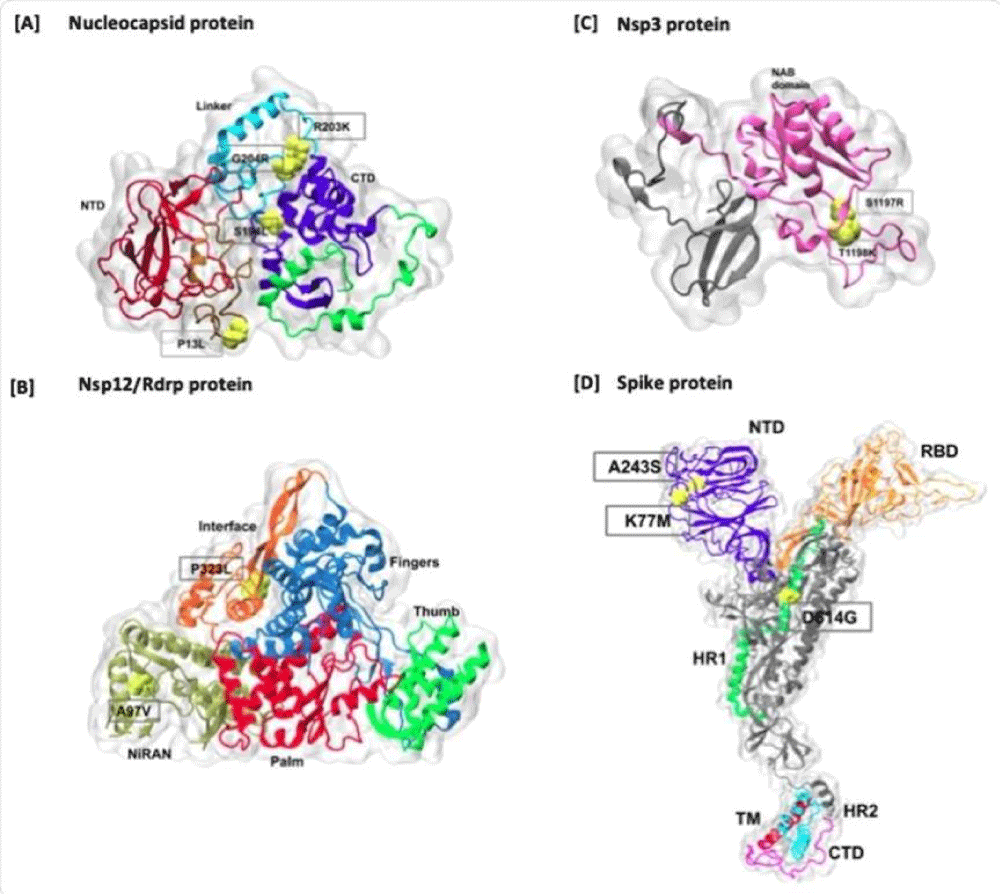
“Mapping of higher frequency amino acid mutations on Nucleo-capsid and Spike proteins. The mutations are marked in red color on the surface representation of each protein. In the Spike protein, all the domains are highlighted in different colors, including NTD, RBD, HR1, Fusion peptide region, Hr2, TM, and CT domains. The cleavage sites are also marked onto the structure
Mutations and Protein Effects:
The investigators also looked at the mutant- proteins expressed as a result of amino- acid AA substitutions across the 104 genomes. There were 53 point of mutations, of which 29 resulted in missense- mutations” (Liji Thomas)(Figuyre 3).
Figure 3: The abnormal convalescence or resolution phase following COVID-19 illness: delayed convalescence, persistence of symptoms, and emergence of symptoms related to organs affected, and prolonged recovery and incapacitating complications and sequelae.
When studied in relation to the proteins affected, the scientists found that most variations were in non-structural protein (nsp) 6, in 68 genomes, with nsp 12 in 65, nsp 3 in 62, and P13L, a nucleocapsid protein, in 53. One of the commonly found worldwide- mutations, D614G, in spike- protein, was found in only 26 genomes.
The researchers and scientist then followed the frequency of mutation with respect to the type of change in the amino- acid AA. They found that in about 44%, the amino acid was not changed, which indicates that perhaps the mutation caused a slight- change in the shape or function of the protein. The same thing was observed with very frequently found mutations, like as P13L.
In some other kid of mutations, the type of amino acid AA was quite different, with a hydrophobic-to-polar or charged type of alteration. One example is that of the addition of a charged residue to the frequently mutated position T1198K in nsp3, or the loss of a charged- group with the important spike- protein SPM- mutation D614G. Such a changeover to a residue with a positive- charge may possibly result in more significant effects on the structure and function of the protein involved. (From By Dr. Liji Thomas, MD 2020 SARS-CoV-2 genome variants in India).
With an observational and visual method a literature search was performed in biomedical database.
Some relevant figure are presented to better explain the meaning of this work.
An experimental hypothesis is submitted in order to verify what fended in literature research.
After this phases a global conclusion is submitted to the researcher to be more verified in larger scale.
From literature
According A Ducatti, et al: “Mutagenic activity of organic- extracts of airborne particulate matter- PM at four different sites within the urban area of the city of Porto- Alegre, Brazil, was investigated using the Salmonella/microsome assay, with the Kado micro-suspension method. The extracts were obtained by sonication, sequentially extracted according to polarity, with cyclohexane (CX) and dichloromethane (DCM) solvents. The different fractions were tested for mutagenicity with the Salmonella typhimurium strains TA98, TA98NR and TA98/1,8-DNP6, without S9 mix metabolic activation. A positive (+) frameshift mutagenic response was observed for non-polar (CX) and/or moderately polar (DCM) compounds at the different sites. The responses varied at different year seasons, and the highest revertants per m3 (rev/m3) values were observed at the site subject to the strongest influence of automotive- vehicles (site 3) in spring (17.13 rev/m3) in DCM fractions, and in summer (13.01 rev/m3) in CX fractions. The responses observed for the TA98NR and TA98/1,8-DNP6 strains suggest the contribution of nitro-compounds to the mutagenic activity observed. Although there appears to be an indicative association between the increased mass per unit volume of air (TSP) and the mutagenicity of organic- extracts of airborne particulate matter in the present study, the Salmonella/microsome assay was a sensitive method to define areas contaminated by genotoxic- compounds, even in samples that present TPS values acceptable by the environmental- quality standards established by law”[1].
Erdinger L, et al: “Among other substances, sulphur dioxide (SO2), nitric oxide (NO) and nitrogen dioxide (NO2) are parameters which are routinely measured to describe the basic air -quality. Organic extracts of airborne particulate matter PM contain mutagenic chemical compounds of different origins. The aim of the study was to find correlations between routine- monitoring data and mutagenic activity of organic extracts of simultaneously drawn samples.
Specimens were collected over a period of 2 years at 8 sampling sites in south-west Germany. Simultaneously, concentrations of NO, NO2, and SO2 were measured on-line within the framework of the official air monitoring network of Baden-Württemberg, Germany. Dust samples were collected for biotesting using high volume air -samplers equipped with glass fibre filters. After sampling was completed, filters were extracted and samples were prepared for biological testing. Mutagenic activity was tested by means of the plate incorporation assay (Ames test) using S. typhimurium TA98 and TA100 tester strains. During the first year of the study, all tests have been performed with and without metabolic- activation. A series of tests has been performed in parallel with TA98 and TA98NR.
Comparison of Ames- test data obtained with and without metabolic- activation indicates no statistically significant difference between the both methods. During the second year of the study, all tests have been performed without metabolic activation. Average- yearly activities at the sampling sites were between 1 and 27 Revertants per m3 (Rev/m3). High -activities were preferably found at congested sites (Karlsruhe, up to 95 Rev/m3). Peak values of over 100 Rev/m3 were found in other places where pollution by traffic is significantly lower. The reason for these high level values is not evident. Tests performed using TA98NR tester strain indicate a significant share (average 31%) of compounds requiring activation by nitro-reductase for mutagenic activity. Average mutagenic- activity can be correlated to routine monitoring parameters. Comparison of averaged data for particular sampling sites indicates significant correlation between nitric- oxide and mutagenic activity in TA98 (r2 = 0.90), while correlation between nitrogen dioxide (0.84) or sulphur dioxide (0.52) and mutagenic -activity is weaker. For TA100, correlations are generally weaker than for TA98. Comparison of data for mutagenic activity and routine monitoring data of distant sites being sampled simultaneously shows parallel- behaviour.Results from this study show that mutagenic- activity can be compared to seasonal and local variations of gaseous indicator air pollutants. Tester strain TA98 generally shows the best correlations. Although pollution by particle-bound mutagenic substances is significantly higher during the cold -season than during summer on average, mutagenic- activity of airborne dust is not a continuous effect. During winter, peak levels as well as low pollution periods can occur. Even during winter time mutagenic activity can reach very low- levels typical for summer-time. Comparison of results for distant sampling sites where samples have been collected simultaneously indicate that ‘classical’ indicators of air- pollution and bacterial mutagenicity of organic- extracts from airborne particulate matter are influenced by connected effects. Seasonal trend of mutagenic activity, in particular, is similar to the concentrations of nitrogen- oxide. NO is a strong indicator for vehicle exhaust gases. It is concluded that the average mutagenic- activity at particular sites can be estimated using NO- concentrations as an indicator” [2].
“The standard sex-linked recessive lethal test was used to test whether NO2 induces lethal- mutations in male germ- cells of Drosophila in the presence or absence of alkylureas. Methylurea, ethylurea and NO2 alone did not enhance the mutation frequency significantly. Highly significant enhancement in mutation frequency was observed when adult flies were exposed to NO2 (150 - 280 ppm) for 3 h after ingestion of methylurea (0.1 M) or ethylurea (0.1 M) for 2 days. Oral- administration of ethylnitrosourea and also of methylurea or ethylurea that had been exposed to NO2 in vitrowere more effective in increasing the mutation frequency than methylurea or ethylurea combined in vivo with NO2. These results suggest that ingested alkylurea is converted in vivo by inhaled- NO2 to highly mutagenic nitrosoalkylurea and/or other mutagens. No significant enhancement of the mutation- frequency was observed when flies were fed on methylurea solution after they had been exposed to NO2” [3].
Mutat Res Oct-Nov 1982
Mutagenicity of airborne particles
H Fukino, S Mimura, K Inoue, Y Yamane
“The mutagenicity of airborne- particles was studied in the Ames Salmonella -system. The mutagenic activity of benzene extracts from airborne- particles was more active in strain TA98 in the presence and absence of S9 mix than in strain TA100. The presence of mutagens, other than benzo[a]pyrene (B[a]P), which did not require S9 mix, was indicated. The monthly variation of direct-acting mutagenic activity showed a pattern similar to that of B[a]P at atmospheric- concentration. The monthly variations of atmospheric NO, NO2, NO-2 and NO-3 concentrations were also similar to that of the direct-acting mutagenic- activity. Atmospheric concentrations of heavy metals such as Pb, Zn, Cd, V and Cu were also found to be related to direct-acting mutagenic activity. These results suggest that emissions from automobiles, home heaters and power plants and so on. May be a primary source of atmospheric, direct-acting mutagens. It is suggested that secondary direct-acting mutagens might be partly formed by the nitration of polycyclic- aromatic hydrocarbons (PAH) with NO2 in the atmosphere because concentrations of B[a]P, NO2 and NO-3 increased simultaneously when the highest direct-acting mutagenic activity was observed” [4].
Yet, pollution is the largest environmental cause of disease and death in the world today, responsible for an estimated about 9 million premature deaths” stated The Lancet Commission in 2017. This phenomenon represents an alarming- threat for human health, as a major cause of respiratory and cardiovascular CV pathologies as well as infertility. Those atmospheric pollutants have severe impacts on the environment and participate in climatic change, acidification, eutrophication, and ecosystem disturbances. Several international and national organizations (WHO, EEA, and INERIS) aim at reducing the global emission of pollutants, thanks to environmental policies as Kyoto and Gothenburg protocols signed in 1997 and 1999, respectively. After that, an encouraging decrease in air pollutants’ levels was measured between 2000 and 2015. Atmospheric- pollutants can be classified into four families: classical, indoor, and organic or inorganic air pollutants. Among the classical ones, which are the principle in amount, sulfur dioxide (SO2), particle matter, ozone (O3), and nitrogen oxide (NOx) species are found. The term NOx refers to a wide range of nitrogen-derived compounds, where nitric oxide (NO) and nitrogen dioxide (NO2) are predominant. Those compounds can be naturally produced at low level by lightnings [4] and volcanic eruptions. NOx is mainly generated by anthropogenic activity (road transport, energy production, industry, and agriculture.
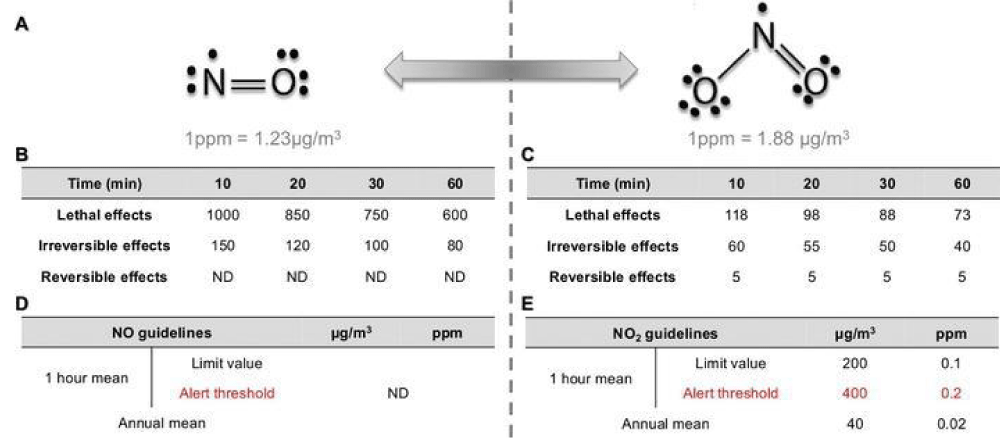
NO and NO2, thanks to their chemistry properties mentioned earlier, are highly diffusible through living membranes and exhibit a strong reactivity [5] (Figures 4-6).
Figure 4: Effect of NO2.
Figure 5:
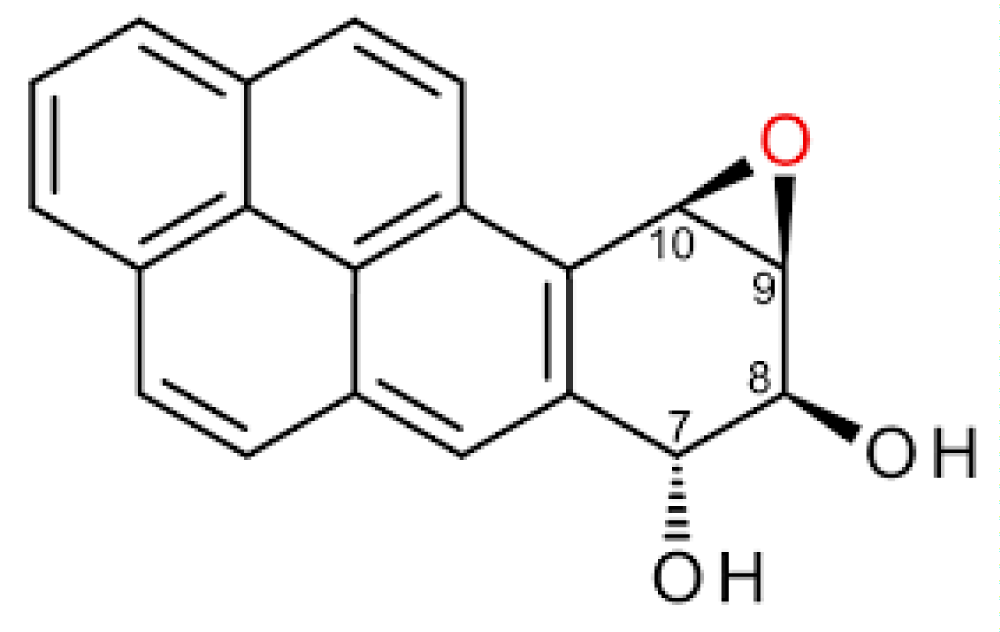
Figure 6: Benzo – a- pirene.
Related effect of air pollution by benzo -a- pirene
“The purpose of this research study was to reveal the pollution characteristics of ambient PM2.5–bound benzo[a]pyrene (B[a]P) in Beijing and to assess the lifetime cancer- risk from respiratory pathway exposure. The average daily dose was evaluated by the annual concentrations of ambient PM2.5–bound B[a]P, combined with Chinese human exposure factors and the age sensitivity factors. The 70-year lifetime cancer risks of different groups were assessed by the exposure assessment and stochastic analysis -method recommended by California Environmental Protection Agency CEPA. The groups were divided by age and gender. The results showed that the ambient PM2.5–bound B[a]P concentration during the cold season was 15.7 times greater than that during the warm season in Beijing. The annual -average concentrations of PM2.5–bound B[a]P in outdoors and indoors were 1.67 ng/m3 and 1.04 ng/m3, respectively, which exceeded the limit of Chinese National Ambient Air- Quality Standard. The cancer- risks of PM2.5–bound B[a]P in males, females, and the general -population were 9.085 × 10−6, 8.05 × 10−6, and 8.740 × 10−6, respectively. The cancer- risk constituent ratios of ambient PM2.5–bound B[a]P in early life (≤16 years of age) for males, females, and the general population were 70.9%, 71.4%, and 71.3%, respectively. The males’ cancer risk of PM2.5–bound B[a]P in Beijing was higher than that of the females. The early- life cancer risk exposure to PM2.5–bound B[a]P should be paid more attention” [6].
Remington KM, et al: in article “Highly mutagenic bypass synthesis by T7 RNA polymerase of site-specific benzo[a]pyrene diol epoxide-adducted template DNA”.
“We have previously developed an in vitro- system IVS that allows quantitative evaluation of the fidelity of transcription during synthesis on a natural template in the presence of all four nucleotides. We have employed this system using a TAA ochre codon reversion- assay to examine the fidelity of transcription by T7 RNA polymerase past an adenine residue adducted at the N6-position with (-)-anti-trans- or (+)-anti-trans-benzo[a]pyrene diol epoxide (BPDE). T7 RNAP was capable of transcribing past either BPDE isomer to generate full-length run-off transcripts. The extent of bypass was found to be 32% for the (-)-anti-trans-isomer and 18% for the (+)-anti-trans-isomer. Transcription past both adducts was highly- mutagenic. The reversion frequency of bypass synthesis of the (-)-anti-trans-isomer was elevated 11,000-fold and that of the (+)-anti-trans-isomer 6000-fold, relative to the reversion frequency of transcription on uneducated- template. Adenine was misinserted preferentially, followed by guanine, opposite the adenine adducted with either BPDE isomer. Although base substitution errors were by far the most frequent mutation on the adducted template, three- and six-base deletions were also observed. These results suggest that transcriptional errors, particularly with regard to damage bypass, may contribute to the mutational- burden of the cell.” [7].
Experimental hypotesys project
In order to verify the effects of someaereosol microenvironment it is possible to think to observe effect in various vials containing in equal way:
Particulate matter, benzo- a pyrene and other air pollutant in an antmosphere rich in NO2 (and like very polluted industrial city examples).
Different vial containings in separated way.
Animal cells; Bacterial cells; Virus DNA particles, Virus RNA particle. (Plus eucaritota cell or not to verify effect of RNA virus translation).
Time of exposition: from 30 days to 6 month.
Other condition: various level of humidity and ozone, and under different level of sun light irradiation (but also in similar way like the sun light in a ozone layer loss region), lightning or not.
After this sample experiment the data must be collected and statistically analyzed to verify level of mutation of genetic materials.
From literature observed many mutation are observed in COVID-19 this involved nucleocapside and spike protein) and this are responsible in increased transmission and severity level of the disease.
Spontaneous phenomena are responsible in many RNA virus evolution and also by environmental pressure.
But it is interesting to observe that some air pollutants like NO2 and particulate matter (and carried substatie like benzo-a-pyrene) can produce a mutagen micro-environment that can contribute in emerging of new variants.
Mutagens that can act also as indirect factor (involving a eucariote translation of information).
In some current decontamination sterilization procedure are used UV ray that with a physical way produced irreversible damages to genetic materials of bacteria and virus so it is possible to consider that mutant agent that currently produces damages in human cells (and cell death) they can produce damages also in Rna -virus.
So it is possible to submit to the researcher the conclusion that an airborne microenvironment with mutagen agent inside can produce new variants also in some RNA -virus expression in higher way vs. without mutagen agent presence (direct or in indirect way).
It must to be considered the complex of RNA virus, Particulate matter covered with mutagen susbtantie and pulmonary cell infected involved in virus reproduction -replication (Figures 7,8).
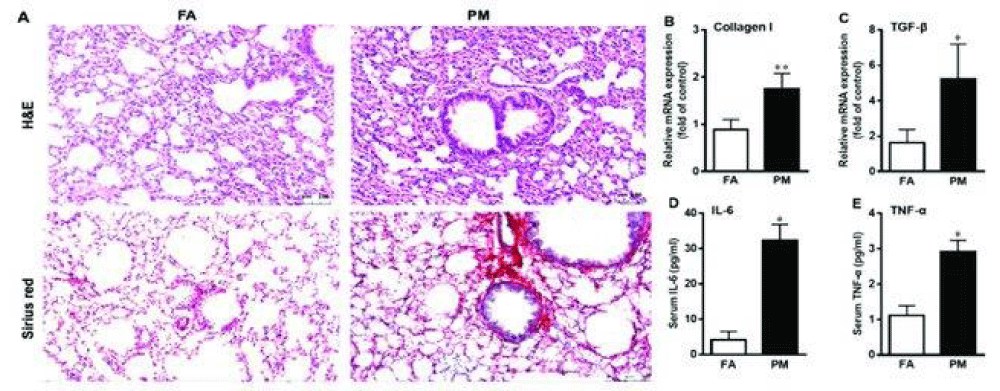
Figure 7: PM2.5 exposure induces pulmonary- inflammation and fibrosis. (A) Lung sections from filtered -air (FA) and particulate matter (PM2.5)-exposed mice were stained with hematoxylin and eosin (H&E) and Sirius red staining at 200× magnification. (B-E) After exposure for 12 weeks, the mRNA- levels of fibrotic- genes collagen I and transforming growth factor β (TGF-β) in lungs (B, C) and the level of inflammatory factors- interleukin 6 (IL-6) and tumor necrosis- factor α (TNF-α) in serum (D, E) were measured by qPCR and enzyme linked immunosorbent- assay (ELISA) kits, respectively. Data are presented as mean ± standard deviation (SD); *p < 0.05, **p < 0.01 vs. FA group. Form TMT-Based Quantitative Proteomics Analysis Reveals Airborne PM2.5-Induced Pulmonary Fibrosis Dec 2018 International Journal of Environmental Research and Public Health 16(1):98 Follow journal DOI: 10.3390/ijerph16010098. Image from Vol-26, Number 9—September 2020 Synopsis.
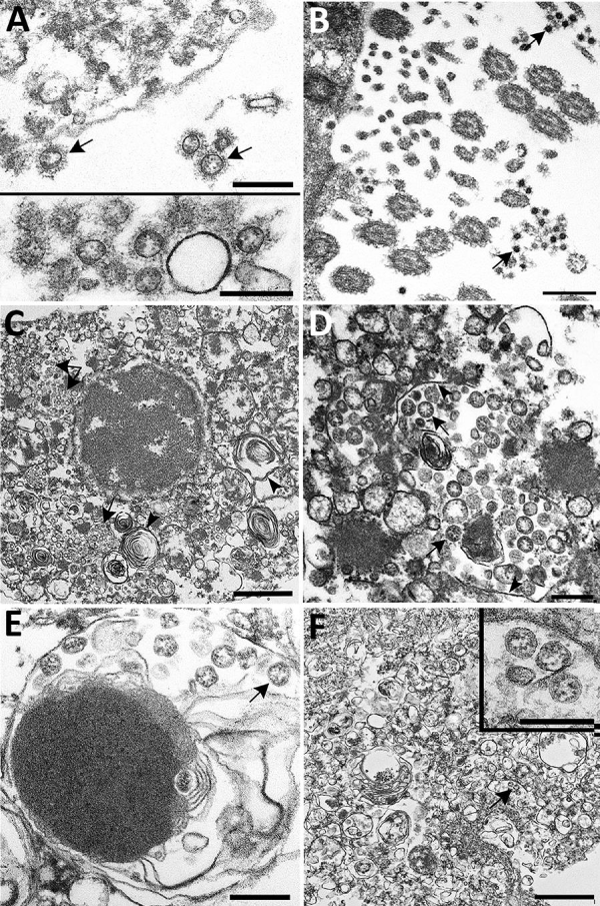
Figure 8: Ultrastructural- features of severe acute respiratory syndrome – SARS coronavirus 2 lung- infection in fatal coronavirus disease. A) Top: alveolar space containing extracellular virions (arrows) with prominent surface projections. Bottom: cluster of virions in the alveolar- space ( A. S.). Scale bars indicate 200 nm. B) Extracellular virions (arrow) associated with ciliated cells of the upper airway. Scale bar indicates 200 nm. C) Membrane-bound vacuoles (arrows) containing viral- particles within the cytoplasm of an infected type II pneumocyte; surfactant (lamellated -material) indicted by arrowheads. Scale bar indicates 1 μm. D) Membrane-bound vacuole (double-headed arrow in panel C) containing virus -particles (arrows) with the characteristic black dots that are cross-sections through the viral nucleocapsid. Arrowheads indicate vacuolar- membrane. Scale bar indicates 200 nm. E) Viral particles (arrow) within a phagosome of an alveolar macrophage. Scale bar: 200 nm. F) Viral particles within a portion of a hyaline- membrane. Scale bar indicates 800 nm. Inset: Higher magnification of virus- particles indicated by arrow; scale bar indicates 200 nm. Image from Vol-26, Number 9—September 2020 Synopsis.
Pathology and Pathogenesis of SARS-CoV-2 Associated with Fatal Coronavirus Disease, United States, Roosecelis B Martines, et al.
Finally: Relevant for this phenomena are: time of exposition, kind of mutagen molecule, carriers, aeresols microenviroment, direct or indirect damage, reversible or irreversible action, efficiency of variant in virus survivor and in transmission.
Observing the literature reported a conclusion can be submitted to the researcher:
As hypothesis of work it is crucial to investigate the role played by some air pollutant in some world region in produce genetic modification and select respiratory virus new variant.
Before actual pandemia coronavirus was not so dangerous like COVID-19.
A real fact that this virus spread form really polluted area like WU-HAN or other world region like.
North Italy and that new variant was observed in South UK, MANAUS, South Africa.
Ethical consideration: All ethical rules respected.
- Ducatti A, Vargas VMF. Mutagenic activity of airborne particulate matter as an indicative measure of atmospheric pollution. Mutat Res. 2003; 540: 67-77. PubMed: https://pubmed.ncbi.nlm.nih.gov/12972059/
- Erdinger L, Dürr M, Höpker KA. Correlations between mutagenic activity of organic extracts of airborne particulate matter, NOx and sulphur dioxide in southern Germany: results of a two-year study. Environ Sci Pollut Res Int. 2005; 12: 10-20. PubMed: https://pubmed.ncbi.nlm.nih.gov/15768736/
- Inoue H, Fukunaga A, Okubo S. Mutagenic effects of nitrogen dioxide combined with methylurea and ethylurea in Drosophila melanogaster. Mutat Res. 1981; 88: 281-290. PubMed: https://pubmed.ncbi.nlm.nih.gov/6789194/
- Fukino H, Mimura S, Inoue K, Yamane Y. Mutagenicity of airborne particles. Mutat Res. 1982; 102: 237-247. PubMed: https://pubmed.ncbi.nlm.nih.gov/6755233/
- Depayras S, Kondakova T, Heipieper HJ, Feuilloley MGJ, Orange N, et al. The Hidden Face of Nitrogen Oxides Species: From Toxic Effects to Potential Cure? Open access peer-reviewed chapter. 2018.
- Chang J, Tao J, Xu C, Li Y, Li N, et al. Pollution characteristics of ambient PM2.5–bound benzo[a]pyrene and its cancer risks in Beijing. Sci Total Environ. 209; 654: 735-741. PubMed: https://pubmed.ncbi.nlm.nih.gov/30448664/
- Remington KM, Bennett SE, Harris CM, Harris TM, Bebenek K. Highly mutagenic bypass synthesis by T7 RNA polymerase of site-specific benzo[a]pyrene diol epoxide-adducted template DNA. J Biol Chem. 1998; 273: 13170-1316. PubMed: https://pubmed.ncbi.nlm.nih.gov/9582358/
- Luisetto M. Deforestation, air pollution and Brasisliant covid-19 variant. Preprint Researchgate. 2021.