More Information
Submitted: 17 July 2020 | Approved: 23 July 2020 | Published: 24 July 2020
How to cite this article: Hamida ME, Raja SM, Seyoum Y, Elkhidir IM, Tekle F. Serological and virological profile of patients with chronic hepatitis B infection in Eritrea. Int J Clin Virol. 2020; 4: 096-101
DOI: 10.29328/journal.ijcv.1001022
Copyright License: © 2020 Hamida ME, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hepatitis B virus (HBV); Chronic Hepatitis B (CHB); Natural history; HBV DNA viral load; Low replicative phase; ELISA; PCR
Abbreviations: ALT: Alanine Aminotransferase; Anti-Hbc: Antibody to Hepatitis B Core Anti- Gen; Anti-Hbs: Hepatitis B Surface Antibody; AST: Aspartate Transaminase; CHB: Chronic Hepatitis B; ELISA: Enzyme-Linked Immunosorbent Assay; Hbeag: Hepatitis B E Antigen; Hbsag: Hepatitis B Surface Antigen; HBV: Hepatitis B Virus; HCC: Hepatocellular Carcinoma; IQR: Interquartile Range; LC: Liver Cirrhosis; M: Mean; Md: Median; Min.: Minimum, Max.: Maxi- Mum; NHL: National Health Laboratory; OCMHS: College of Medicine and Health Sciences; SD: Standard Deviation; WHO: World Health Organization
Serological and virological profile of patients with chronic hepatitis B infection in Eritrea
Mohammed Elfatih Hamida1*, Saud Mohammed Raja2, Yemane Seyoum2, Isam Mohammed Elkhidir3 and Freweini Tekle4
1Orotta College of Medicine and Health Sciences, Department of Microbiology, Asmara, Eritrea
2Orotta College of Medicine and Health Sciences, Department of Internal Medicine, Asmara, Eritrea
3University of Khartoum, Faculty of Medicine, Department of Microbiology, Khartoum, Sudan
4Ministry of Health, National Health Laboratory, Asmara, Eritrea
*Address for Correspondence: Mohammed Elfatih, Orotta College of Medicine and Health Sciences, Department of Microbiology, Asmara, Eritrea, Email: [email protected]
Background: Hepatitis B virus infection is a major cause of liver associated morbidity and mortality with diverse spectrum of disease. It is estimated about 15% to 40% of patients with hepatitis B virus infection progress to chronic hepatitis and about 15% to 25% die from disease complications. The main aim of this study was to evaluate the serological and virological markers of patients with chronic hepatitis B virus infection to determine the natural history of chronic hepatitis B infection in the Eritrean setting.
Methods: A laboratory-based cross-sectional study was conducted on 305 patients with HBsAg positive who presented to Orotta National Referral Hospital, Halibet Hospital, Sembel Hospital and National Health Laboratory in Asmara, Eritrea from January 2017 to February 2019. Enzyme-linked immunosorbent assay was performed to detect hepatitis B serological markers (anti-HBc, HBsAg, anti-HBsAb, HBeAg and anti-HBeAg). Hepatitis B DNA viral loads and liver transaminase levels were determined. Data analysis was conducted using SPSS version 25.0.
Results: A total of 305 patients presented with HBsAg positive serology with a mean age of 41.3 (± 13.7) years ranging from 16 to 78 years. Males were 218 (71.5%) and females 87 (28. 5%).Anti-HBc was positive in 300 (98.4%), of which 293 (97.5%) were positive for HBsAg and 7 (2.3%) positive for anti-HBs. Among these 293 patients, 20 (6.8%) were HBeAg positive/anti-HBe positive, 242 (82.6%) HBeAg-negative/anti-HBe-positive and 31 (10.6%) were HBeAg negative/anti-HBe-positive. Detectable HBV DNA was found in 122(41.6%) of the 293 cases. Alanine transaminase was normal in 90% of HBeAg-positive and in 91.2% of HBeAg-negative patients. Hepatitis B DNA viral load was >2,000 IU/mL in 67 (22.86%) and >200,000 IU/mL level was more frequently detected in HBeAg positive (20.0%) compared to HBeAg negative (1.8%) subjects (p < 0.001).
Conclusion: This study shows predominance of HBeAg-negative and low replication phase of HBV infection among patients in Eritrea. It also documented that most patients had chronic infection with normal liver transaminase levels in the absence of biochemical signs of hepatitis. This study will provide a basis for therapeutic evaluation of patients and planning national treatment guidelines in the Eritrean setting.
Hepatitis B virus (HBV) infection is a major global public health threat especially in developing countries. The World Health Organization (WHO) estimates that in 2015, about 60 million Africans were living with chronic HBV infection [1]. Most African countries fall within the high endemicity regions [2]. Infection with HBV is usually acquired through perinatal or childhood exposure to the virus, contaminated blood transfusions or unprotected sexual contact [3] and progresses to long-term HBV infection in about 15% to 40% of cases depending on viral and host factors [4,5]. Although chronic HBV infection is commonly asymptomatic, an estimated 15% to 25% of patients will die from disease progression and complication such as liver cirrhosis (LC) and hepatocellular carcinoma (HCC)[4].
The natural history of chronic HBV infection is categorized into five clinical phases according to Hepatitis B e Antigen (HBeAg), HBV DNA levels, alanine aminotransferase (ALT) and liver inflammation status. These are (1) immune tolerant HBeAg positive phase, which progresses to (2) immune-reactive HBeAg-positive phase, (3) inactive HBV carrier state (HBeAg negative) phase, (4) HBeAg-negative chronic hepatitis B (CHB) phase and (5) HBsAg negative phase [6,7]. However, not all chronically infected patients go through all these phases and the phases vary in duration. It is quite a complex process and is difficult to distinguish between these phases in clinical practice [8]. Therefore, different periodic studies for HBV serological and viral markers studies are required to assess treatment requirement.
Patients with HBV infection should be screened serologically to determine whether they have chronic infection or had suffered previous exposure to HBV. To that ends, it is necessary to test the serum for hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (anti-HBs), and antibody to hepatitis B core antigen (anti-HBc) [9].
The quantification of HBV DNA (viral load) level is the most reliable virological marker to determine active HBV replication and, in combination with liver enzymes and clinical assessment, it enables accurate evaluation of natural history of hepatitis B, as well as the risk of disease progression among HBV infected patients [10-12]. Therefore, understanding of these markers is quite crucial in monitoring the disease progression and optimal management of patients [13].
Eritrea is geographically located in sub-Saharan Africa, a region considered endemic to HBV infection with an intermediate to high prevalence [1]. The epidemiology of HBV infection in Eritrea is not well documented and there are only limited studies conducted to explore the extent of the problem. Available HBV infection prevalence data from previous studies conducted reported an intermediate prevalence of HBsAg seromarkers of 2.6% to 3.2% [14,15]. However, the limited of availability of the serological and molecular testing constitutes one of the most important barriers for obtaining a clearer picture. These drawbacks pose a challenge for clinicians in determining the exact clinical stage of HBV infected patients and assessment of requirement for treatment. Such barriers in resource limited countries may hinder the attainment of global viral hepatitis control [1]. We performed this study to determine HBV serological and virological markers to investigate the natural history of CHB infection among Eritrean patients.
Study design and setting
A laboratory-based cross-sectional study was conducted among 305 patients with seropositivity for HBsAg attending to the hepatology units and outpatient clinics of Orotta National Referral Hospital, Halibet Hospital, Sembel Hospital and National Health Laboratory (NHL) in Asmara, Eritrea, between January 2017 and February 2019. Patients with acute hepatitis and blood donors who tested positive for HBsAg were not included in the study.
Specimen’s collection
Venous blood (5 ml) was collected aseptically by a trained phlebotomist into a dry tube. The sample was then centrifuged at 3000 rpm for 5 minutes at room temperature to obtain serum that was divided into two cryogenic tubes — one for serology and biochemistry and the second for molecular testing kept frozen at - 20 oC until testing.
Serological tests
Serological markers for hepatitis B virus (anti-HBc total, HBsAg, anti-HBsAg HBeAg and anti-HBeAg) were performed by using commercial enzyme-linked immunosorbent assay (ELISA) in vitro diagnostic kits (Fortress Diagnostics, United Kingdom). The concentration of the specific antibody–antigen complex in the specimen was determined by means of a cut‑off value according to the procedures described by the manufacturers. In addition, positive and negative controls were run simultaneously to check the validity of test. All patients were tested for antibody to HBV core antigen (anti-HBc) total. Those with detectable anti-HBc were tested for HBV surface antigen (HBsAg) and antibody to HBV surface antigen (anti-HBs). Those with detectable antibody to HBV core antigen (anti-HBc) and HBV surface antigen (HBsAg) were tested for hepatitis B ‘e’ antigen (HBeAg) as well as antibody to hepatitis B ‘e’ antigen (anti-HBe). All specimens with positive HBsAg and positive for anti-HBc were further processed for quantitative HBV DNA viral load.
Biochemistry
Serum liver transaminase levels, alanine transaminase (ALT) and aspartate transaminase (AST), were measured by using automated clinical chemistry analyzer (Beckman coulter au480, USA) following the manufacturer’s instructions, in accordance with principle based on International federation of clinical chemistry and quality control measures. The normal reference value for ALT and AST were considered as <40 U/l[16].
HBV viral load quantification
Quantitative detection of HBV DNA (viral load) in serum of CHB patients was measured using COBAS AmpliPrep/COBAS TaqMan 48HBV Test, version 2.0 (Roche Diagnostics, Switzerland) for automated amplification and detection of a limit between 20 to 170,000,000 IU/mL. Specimen preparation and simultaneous polymerase chain reaction (PCR) amplification of target DNA was done followed by detection of cleaved dual‑labelled oligonucleotide detection probe specific to the target according to the manufacturer’s instructions. HBV viral load quantitative level exceeding 2,000 IU/mL was considered a high‑level HBV replication whereas below 2,000 IU/mL as low‑level replication [12]. An undetectable viral load was defined as HBV DNA below the level of sensitivity of the assay (<20 IU/mL).
Statistical analysis
Data were analyzed using SPSS (Statistical Package for the Social Sciences) version 25.0 (IBM; Chicago, IL, USA) and the values were represented in numbers, percentages and mean ± standard deviation (SD). Statistical analyses for categorical variable were performed to determine test of significance. A p value < 0.05 was considered as statistically significant.
Ethical approval and consent
Ethical approval for this study was obtained from ethical research committee board of Orotta College of Medicine and Health Sciences (OCMHS) and Ministry of Health state of Eritrea (MoH). A written informed consent was obtained from each study participant before collecting the demographic and clinical data.
Socio-demographic characteristics
Of the 305 patients who presented with a positive HBsAg serum, 218 (71.5%) were males and 87 (28.5%) were females. Mean age ± standard deviation (SD) was 41.39 ± 13.77 years ranging from 16 to 78 years old. Most (27.0%)of the participants were in the age group of 41- 50 years, followed by equal distribution of 51 and above group and 31-40 years’ group were (23.5% each). Most (70.8%) of the participants were married. The majority (39.0%) of participants were government employees followed by nearly equal distribution of private workers (27.5%) and unemployed (26.9%). Almost half of the patients (49.8%) hailed from Zoba Maekel, the central region of the country where the hospitals in this study and the national health laboratory are located. All these characteristics and distributions by gender are as shown in table 1.
| Table 1: Socio-demographic characteristics of HBV infected patients (n=305). | |||
| Variable | Gender* | Total** n (%) |
|
| Male n (%) | Female n (%) | ||
| Age (M = 41.39 ± SD = 13.77, Md = 40.0 ± IQR = 19, Min.= 16, Max.= 78) | |||
| Less than 20 | 8 (66.7) | 4 (33.3) | 12 (4.1) |
| 21 to 30 | 47 (73.4) | 17 (26.6) | 64 (21.8) |
| 31 to 40 | 45 (65.2) | 24 (34.8) | 69 (23.5) |
| 41 to 50 | 58 (73..4) | 21 (26.6) | 79 (27.0) |
| 51or above | 55 (79.7) | 14 (20.3) | 69 (23.5) |
| Marital Status | |||
| Single | 59 (75.6) | 19 (24.4) | 78 (25.6) |
| Married | 154 (71.3) | 62 (28.7) | 216 (70.8) |
| Divorced | 5 (45.5) | 6 (54.5) | 11 (3.6) |
| Occupation | |||
| Government employee | 94 (79.0) | 25 (21.0) | 119 (39.0) |
| Unemployed | 36 (43.9) | 46 (56.1) | 82 (26.9) |
| Private workers | 75 (89.3) | 9 (10.7) | 84 (27.5) |
| Students | 13 (65.0) | 7 (35.0) | 20 (6.6) |
| Zoba | |||
| Anseba | 11 (64.7) | 6 (35.3) | 17 (5.6) |
| Debub | 43 (66.2) | 22 (33.8) | 65 (21.3) |
| Gash Barka | 32 (72.7) | 12 (27.3) | 44 (14.4) |
| Maekel | 110 (72.4) | 42 (27.6) | 154 (49.8) |
| Semienawi Keiyh Bahri | 14 (82.4) | 3 (17.6) | 17 (5.6) |
| Debubawi Keiyh Bahri | 8 (80.0) | 2 (20.0) | 10 (3.3) |
| Hospital | |||
| National Health Laboratory | 152 (72.2) | 50 (24.8) | 202 (66.2) |
| Orotta Hospital | 46 (63.9) | 26 (36.1) | 72 (23.6) |
| Halibet Hospital | 13 (68.4) | 6 (31.6) | 19 (6.2) |
| Sembel Hospital | 7 (58.3) | 5 (41.7) | 12 (3.9) |
| Total | 218 (71.5%) | 87 (28.5%) | 305 (100%) |
| M: Mean; SD: Standard deviation; Md: Median; IQR: Interquartile Range; Min.: Minimum; Max.: Maximum *Percentage is computed from the row total, **Percentage is computed from total liver patients. |
|||
HBV serology
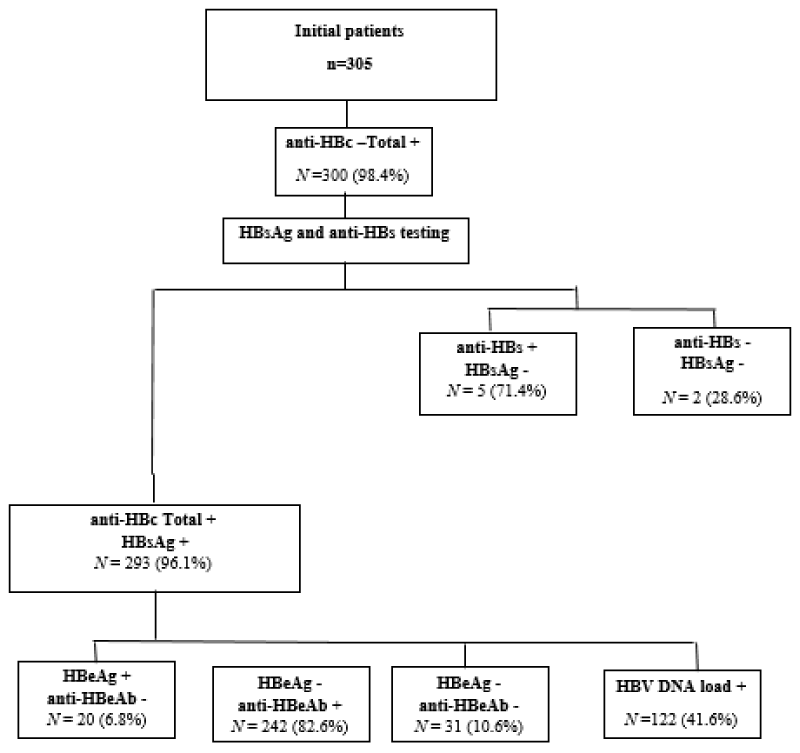
With the exception of 5 cases, 300 (98.4%) out of the 305 initially positive for HBsAg were found to have positive anti-HBc total. Out of the 300 total anti-HBc positive, 97.7% had positive HBsAg with 2.3% having positive anti-HBsAg. From the total 305 of HBV patients, the percentage of anti-HBc total positive/HBsAg-positive was found to be 96.1%. Further investigation of those anti-HBc total positive/HBsAg-positive (n = 293) revealed that the percentage of HBeAg positive/anti-HBeAg negative, anti-HBeAg positive/HBeAg-negative and both HBeAg negative/anti-HBeAg negative were 6.8 %, 82.6% and 10.6% respectively. Overall, HBeAg was detected in 6.8% (20/293) of patients, anti‑HBe was positive in 82% (242/293) (Figure 1). There was no statistically significant difference in the age categories of HBeAg positive patients compared with HBeAg negative patients (p = 0.362). Anti‑HBe was significantly more frequent in HBeAg‑negative patients compared with HBeAg‑positive subjects (p value ≤ 0.001).
Figure 1: Study participant’s distribution flowchart through hepatitis B virus seromarkers./p>
Hence, we started with 305 HBsAg patients by screening their serum for anti-HBc (total) seromarker, 5 were excluded because they tested negative and include who test positive for further tested for both HBsAg and anti-HBs using ELISA, because it is the only available method to confirm the HBsAg positivity patients and the chronicity phase (anti-HBc total Ab positive and HBsAg positive), or the patients with natural immunity (HBsAg negative, anti-HBc (total) Ab and anti-HBs Ab positive) or determine who recovered from previous HBV infection with anti-HBs Ab clearance (HBsAg negative, anti-HBc total positive without anti-HBs Ab).
Biochemistry
According the cut-off of normal value for AST and ALT below 40 U/L, more than 90% of the HBV patients had normal AST and a similar percentage of normal ALT level as shown in table 2. The levels in males and females were similar.
| Table 2: Percentage and distribution of positive HBV markers, Liver aminotransferases and viral load among HBV infected patients by gender. | |||
| Variable | Total n (%) | Gender | |
| Male n (%) | Female n (%) | ||
| HBV seromarkers –positive | |||
| Anti-HBc total | 300 (98.4) | 215 (70.5) | 85 (27.9) |
| Anti-HBsAg | 7 (2.3) | 4 (1.3) | 2 (1.0) |
| HBsAg | 293 (97.7) | 213 (71.1) | 80 (26.7) |
| Anti-HBc total positive/ HBsAg-positive | 293 (96.1) | 213 (69.8) | 80 (26.2) |
| HBeAg-positive/Anti-HBeAg-negative | 20 (6.8) | 15 (5.1) | 5 (1.7) |
| HBeAg-negative/Anti-HBeAg-positive | 242(82.6) | 173 (59.0) | 69 (23.5) |
| Neither HBeAg nor anti-HBeAg | 31(10.6) | 25 (8.5) | 6 (2.0) |
| Liver aminotransferases | |||
| ALT | |||
| Normal (<40 U/l) | 259 (84.9) | 185 (60.7) | 74 (24.3) |
| Elevated(>40 U/l) | 46 (15.1) | 33 (10.8) | 13 (4.3) |
| AST | |||
| Normal (<40 U/l) | 267 (87.5) | 190 (62.3) | 77 (25.2) |
| Elevated (>40 U/l) | 38 (12.5) | 28 (9.2) | 10 (3.3) |
| Viral load (IU/mL) | |||
| ˂20 | 183 (60.0) | 129 (59.2) | 54 (62.1) |
| 21‑ 2,000 | 55 (18.0) | 38 (12.5) | 17 (5.6) |
| 2,001‑ 20,000 | 40 (13.1) | 33 (10.8) | 7 (2.3) |
| 20,000‑ 200,000 | 18 (5.9) | 11 (3.6) | 7 (2.3) |
| ˃200,001 | 9 (3.0) | 7 (2.3) | 2 (0.7) |
| Anti-HBc: Antibody to Hepatitis B core antigen; anti-HBe: antibody to hepatitis B‘e’ antigen; anti-HBs: antibody to hepatitis B surface antigen; HBeAg: hepatitis B ‘e’ antigen; HBsAg: hepatitis B surface antigen. All percentages are computed out of the total of liver disease patients with HBV. |
|||
As HBeAg is a sign of active liver disease, liver transaminases were specifically analyzed according to HBeAg. Out of 20 HBeAg-positive patients, 15 (75.0%) had raised ALT and 5 (25.0%) had normal ALT levels. In case of 273 HBeAg-negative patients 29 (10.6%) were with elevated ALT level and 244 (89.4%) were with normal ALT level (Table 3). There was statistically significant difference in the rate of elevated ALT levels between HBeAg positive patients and HBeAg negative patients (p value = 0.001).
| Table 3: Distribution of age, liver enzymes and HBV DNA levels according to HBeAg status in the patients with positive anti-HBc (total) / HBsAg (n = 293). | |||
| Parameters | HBeAg‑positive, n (%) |
HBeAg‑negative, n (%) | p value |
| Age group | |||
| Less than 20 | 4 (20.0) | 8 (2.9) | 0.003 |
| 21 to 30 | 6 (30.0) | 58 (21.2) | |
| 31 to 40 | 2 (10.0) | 67 (24.5) | |
| 41 to 50 | 5 (25.0) | 74 (27.1) | |
| 51 or above | 3 (15.0) | 66 (24.2) | |
| Anti-HBe | |||
| Positive | 1 (5.0) | 241 (88.3) | < 0.001 |
| Negative | 19 (95.0) | 32 (11.7) | |
| ALT level | |||
| Normal | 5 (25.0) | 244 (89.4) | <0.001 |
| Elevated | 15 (75.0) | 29 (10.6) | |
| Viral load (IU/mL) | |||
| ≤2,000 | 8 (40.0) | 218 (79.9) | < 0.001 |
| 2,001‑ 20,000 | 6 (30.0) | 34 (12.5) | |
| 20,001‑ 200,000 | 2 (10.0) | 16 (5.9) | |
| >200,001 | 4 (20.0) | 5 (1.8) | |
HBV viral load
Of 293 anti-HBc total positive/HBsAg-positive patients, 122 (41.6%) were detected to have HBV viral load more than 20IU/ml, 55 (18 %) patients had HBV viral load between 21 to 2000 IU/ml, 40 (13.1%) had HBV viral load between 2001 and 20,000 IU/ml, 18 (5.9%) had HBV viral load between 20,000 and 200,000, while 9 (3%) had values >200,001. The Overall high‑level viral replication with HBV DNA exceeding 2,000 IU/mL was detected in 67 (22.86%) patients.
The relationship between HBV DNA viral load levels and HBeAg status among patients. Table 3 shows slow replicative activity of HBV viral load (≤2,000 IU/ml) occurred more frequently in HBeAg‑negative patients [218 (79.9%)] than in HBeAg‑positive patients [8 (40.0%)]. HBV DNA replication between 20,001 and 200,000 and levels > 200,000 IU/mL were more frequently detected in HBeAg‑ positive patients (10.0% and 20.0%) compared with HBeAg‑ negative patients (5.9% and 1.8%), respectively. Subsequently, there was a statistically significant difference between HBV DNA levels of HBeAg positive patients in comparison to HBeAg negative patients (p value < 0.001).
Eritrea is an east African multi-ethnic country surrounded by HBV hyperendemic countries. Like in many of sub-Saharan African countries, the serological profile of HBV infection in Eritrea have not been well known and confirmed [1,14]. The dynamics of HBV infection range according to clinical manifestation of the patients from acute infection stage, chronic inactive carrier state, and a chronic progressive liver disease, culminating in liver cirrhosis (LC) and hepatocellular carcinoma (HCC) [17]. Due to limited and high cost of molecular testing, fewer studies are available evaluating both HBV seromarkers and quantification of HBV DNA in infected population [1,18]. Hence, the current study was conducted to evaluate the serological and virological status of HBV infected patients in Eritrea.
Hepatitis B virus infection is known to affect millions of people worldwide cutting across gender and all age groups [19]. Patients who exhibited HBsAg positive serology were included in this study. The patients’ mean age was 41.39 (SD = 13.77) years ranging from 16 to 78 years old. This was congruent to a study by Souza, et al. 2015 and Islam, et al. [20]. Our study showed a preponderance of male participants who constituted 71.5% of the cases compared to 28.5% females. This distribution is in agreement with an Ethiopian study that reported the prevalence of HBV infection being higher in males (38%) than females (31.8%) [21], indicating male predominance and underlying higher prevalence of HBV among males in Africa. Most of the participants were adults above 20 years of age with similar distribution of the age groups. This conforms to the bulk of the literature that shows high prevalence of HBV in both middle-and older aged adults, as compared to young adults. In accordance, Elmukashf, et al. (2012) reported most of the patients (58.4%) were between the age group 30-49 years [22].
The patterns of HBV serological markers play an essential role in characterizing the disease phases and determining requirement for antiviral therapy. In endemic regions of HBV infection estimated that up to one-third of the population have evidence of previous infection with HBV as indicated by an isolated anti-HBc serology [9]. Our findings show high percentage (96.1%) of patients in chronicity phase who test positive for both anti-HBc total and HBsAg, compared to low perseverance (2.3%) of patients who recovered from previous exposure to HBV infection or natural immunity in consistent with a previous study in Eritrea by Fessehaye, et al. [14]. This implies the need to implement and enforce vaccination programs among high and low risk groups [23-25].
This study documented a higher proportion HBeAg negative (93.2%) compared to HBeAg positive (6.8%) patients in congruence with studies such as conducted by Di Bisceglie, et al. [26]. Four out of five our patients were in a low replicative phase of chronic hepatitis B characterized by HBeAg‑negative status, detection of HBe antibodies (anti‑HBe), and DNA concentration below 2000 IU/mL with normal ALT, who are considered as chronic inactive carriers in similar proportion to what some studies have shown [27-30]. The determination of high percentage of HBeAg negative patients with low HBV viral loads in our study implies that most of the HBV patients are not eligible for antiviral therapy due to minimal or absent liver injury with low risk of disease progression [12].
We found high prevalence of HBeAg‑negative hepatitis patients with high‑level active HBV replication despite detectable anti‑HBe antibodies. This finding is similar to studies that have shown HBeAg‑negative hepatitis as the common form of chronic hepatitis B virus associated liver disease progression and activity of HBV viral mutations in Asia and sub‑Saharan Africa [18,31,32].
To the best of our knowledge, this study is the first of its kind in Eritrea to describe the serological and virological markers among patients with chronic hepatitis B infection. Nevertheless, some limitations in the current study are worth mentioning. Being a cross-sectional study without follow-up of the patients, it is difficult to have an accurate picture of the natural history of the infection. The fact that it is laboratory-based lacks important clinical data for categorizing the patients according to their status of liver disease using ultrasonography or liver biopsy.
This study demonstrated the predominance of HBeAg-negative infection and low replication of HBV with significantly low viral loads among HBV patients in Eritrea. Our study also shows that most patients had chronic hepatitis B infection with normal liver transaminase levels in the absence of laboratory sign of hepatitis. Our findings are expected to provide a basis for an informed planning of national chronic hepatitis B treatment guidelines. A large-scale population or clinical cohort study in the future is recommended to fully explore the natural history and phases of hepatitis B infection among Eritrean patients.
Availability of data and materials
All data and materials described in the manuscript are available.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
Mohammed Elfatih Hamida, Saud Mohammed Raja, Yemane Seyoum, Isam Mohammed Elkhidir and Freweini Tekle conceived and de- signed the study. Mohammed Elfatih Hamida, Saud Mohammed Raja and Freweini Tekle analyzed the data. Mohammed Elfatih Hamida, Saud Mohammed Raja and Yemane Seyoum revised the paper. Mohammed Elfatih Hamida and Saud Mohammed Raja wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethical approval for this study was obtained from ethical research committee board of Orotta College of Medicine and Health Sciences (OCMHS) and Ministry of Health state of Eritrea (MoH). A written informed consent was obtained from each study participant before collecting the demographic and clinical data.
We would like to thanks; the members of the Immunoserology department at Eritrean National Health Laboratory (ENHL), and Eritrean National Council for Higher Education.
- WHO. Global hepatitis report 2017. 2017: World Health Organization.
- Kramvis A, Kew MC. Epidemiology of hepatitis B virus in Africa, its genotypes and clinical associations of genotypes. Hepatol Res. 2007. 37: S9-S19. PubMed: https://pubmed.ncbi.nlm.nih.gov/17627641
- Bogler Y, Wong RJ, Gish RG. Epidemiology and Natural History of Chronic Hepatitis B Virus Infection, in Hepatitis B Virus and Liver Disease. Springer. 2018; 63-89.
- Lavanchy D, Kane M. Global epidemiology of hepatitis B virus infection, in Hepatitis B virus in human diseases. Springer. 2016; 187-203. PubMed:
- Yang HC, Shih YF, Liu CJ. Viral factors affecting the clinical outcomes of chronic hepatitis B. J Infect Dis. 2017; 216(suppl_8): S757-S764. PubMed:
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012; 57: 167-185. PubMed: https://pubmed.ncbi.nlm.nih.gov/22436845/
- Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, et al. A ASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016; 63: 261-283. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5987259/
- Croagh CM, Lubel JS. Natural history of chronic hepatitis B: phases in a complex relationship. World J Gastroenterol. WJG. 2014; 20: 10395. PubMed: https://pubmed.ncbi.nlm.nih.gov/25132755/
- Lau GK. How do we handle the anti‐HBc positive patient? (in highly endemic settings). Clin Liver Dis. 2015; 5: 29-31. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6490450/
- Liu J, Yang H, Lee M, Lu S, Jen C, et al. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut. 2014; 63: 1648-1657. PubMed: https://pubmed.ncbi.nlm.nih.gov/24225939
- Kirk GD, Lesi OA, Mendy M, Akano AO, Sam O, et al. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 2004; 39: 211-219. PubMed: https://pubmed.ncbi.nlm.nih.gov/14752840
- WHO. Guidelines for the prevention care and treatment of persons with chronic hepatitis B infection: Mar-15. 2015: World Health Organization.
- Li Q, Ren X, Lu C, Li W, Huang Y, et al, Evaluation of APRI and FIB-4 for noninvasive assessment of significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT≤ 2 ULN: A retrospective cohort study. Medicine. 2017; 96: e6336. PubMed: https://pubmed.ncbi.nlm.nih.gov/28328813/
- Fessehaye ND, Berhane NA, Ahimed H. Prevalence of hepatitis B virus infection and associated seromarkers among pregnant women in Eritrea. J Human Virol Retrovirol. 2018; 6: 00191.
- Fessehaye N, Naik D, Fessehaye T. Transfusion transmitted infections–A retrospective analysis from the National Blood Transfusion Service in Eritrea. Pan African Med J. 2011; 9; 40. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22145069
- Papatheodoridis GV, Manolakopoulos S, Liaw Y, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol. 2012; 57: 196-202. PubMed: https://pubmed.ncbi.nlm.nih.gov/22450396/
- Merican I. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000. 15: 1356-1361.
- Lesi O. Serological and virological markers of nigerian patients with hepatitis B infection. Niger J Clini Practice, 2019; 22: 534.
- Baig S. Gender disparity in infections of Hepatitis B virus. J Coll Physicians Surg Pak, 2009. 19: 598-600. PubMed: https://pubmed.ncbi.nlm.nih.gov/19728952/
- Souza ND. Assessment of health-related quality of life and related factors in patients with chronic liver disease. Bra J Infect Dis. 2015. 19: 590-595.
- Abdelmenan S, Banes A, Berhane Y, Abebe M, Wandall JH. Etiology of Chronic Liver Disease in Ethiopia: A Case Control Study with Special Reference to Viral Hepatitis and Alcohol. EC Gastroenterol Digestive Sys. 2018; 5: 120-128. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6402780/
- Elmukashfi TA, Ibrahim OA, Elkhidir IM, Bashir AA, Elkarim MAA. Socio-demographic characteristics of health care workers and hepatitis B virus (HBV) infection in public teaching hospitals in Khartoum State, Sudan. Glob J Health Sci. 2012; 4: 37-41. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4776931/
- Hoofnagle J, Gerety R, Barker L. Antibody to hepatitis-B-virus core in man. The Lancet. 1973; 302: 869-873.
- Hollinger FB. Hepatitis B virus infection and transfusion medicine: science and the occult. Transfusion. 2008; 48: 1001-1026. PubMed: https://pubmed.ncbi.nlm.nih.gov/18454738/
- Liang X. Reprint of: Epidemiological serosurvey of Hepatitis B in China—Declining HBV prevalence due to Hepatitis B vaccination. Vaccine. 2013; 31: J21-J28. PubMed:
- Di Bisceglie AM, Lombardero M, Teckman J, Roberts L, Janssen HLA, et al, Determination of hepatitis B phenotype using biochemical and serological markers. J Viral Hepatitis. 2017; 24: 320-329. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27917600
- Terrault N. American Association for the Study of Liver D. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016; 63: 261-283.
- Roos R. A cross sectional study of HBeAg negative chronic hepatitis B virus infection in Cape Town, South Africa: P067. J Viral Hepatitis. 2015; 22: 53-54.
- Ivón CFM, Zaily DG, Leda Patricia DCE, Enrique GG, Enrique AS, et al, Current Condition of Chronic Hepatitis B Virus Infection in Cuban Adults. Curr Ther Res Clin Exp. 2017; 85: 15-19. PubMed: https://pubmed.ncbi.nlm.nih.gov/29158854/
- Tufon KA, Anong DN, Meriki HD, Georges, TD, Maurice, M, et al, Characterization and assessment of HBV chronically infected patients: Identification of those eligible for treatment in the South West region of Cameroon. PloS one. 2018; 13: e0203312. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6124766/
- Hadziyannis SJ, VassilopoulosD. Hepatitis B e antigen–negative chronic hepatitis B. Hepatology. 2001; 34: 617-624. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11584355
- Iregbu K, Nwajiobi‑Princewill PI. Viral Load Pattern Among Hepatitis B Surface Antigen‑positive Patients: Laboratory Perspective and Implications for Therapy. Ann Med Health Sci Res. 2016; 6: 95-99. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4866374/