More Information
Submitted: 19 February 2020 | Approved: 27 February 2020 | Published: 28 February 2020
How to cite this article: Deng L, Chu C, Chen X, Wu Z. Pseudoephedrine protects mice from infection of H1N1 virus. Int J Clin Virol. 2020; 4: 014-020.
DOI: 10.29328/journal.ijcv.1001008
ORCiD: orcid.org/0000-0001-6646-0753
Copyright License: © 2020 Deng L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pseudoephedrine; Influenza A virus; Cytokine storm; Replication of influenza a virus
Pseudoephedrine protects mice from infection of H1N1 virus
Li Deng1,2, Chengzhi Chu3, Xiaoyin Chen2* and Zhongping Wu1*
1Teaching & Research Department of Clinical and Classic Medicine, College of Basic Medical Science, Shanghai University of Traditional Chinese Medicine, China
2College of Traditional Chinese Medicine, Jinan University, Guangzhou, China
3Anhui College of Traditional Chinese Medicine, Wuhu, China
*Address for Correspondence: Zhongping Wu, Teaching & Research Department of Clinical and Classic Medicine, College of Basic Medical Science, Shanghai University of Traditional Chinese Medicine, China, Tel: +86 2151323015; Email: [email protected]
Xiaoyin Chen, College of Traditional Chinese Medicine, Jinan University, Guangzhou, China, Tel: +86 2085226197; Email: [email protected]
Ephedra, an ancient herb, is applied to treat common cold and influenza for such a long time in China. Pseudoephedrine is a main active ingredient from Ephedra which is used for relieving nasal congestion clinically. We previously reported that pseudoephedrine showed a potent anti-inflammatory effect other than sympathomimetic effects. In the present study, we aimed to investigate whether pseudoephedrine could protect mice from the H1N1 virus infection. The mice were infected with a 20% LD50 influenza A virus (IAV) suspension via intranasal administration to establish a virus infection model. Further, the mice were orally administered pseudoephedrine or oseltamivir for 4 days from one day after infection. Our results showed that pseudoephedrine improved lung pathological damage during the IVA infection period, and it dramatically increased the survival rate and attenuated loss of body weight compared with the virus-infected control group. In addition, pseudoephedrine inhibited the cytokine storms and mRNAs expression of the TLR7 signaling pathway. Surprisingly, pseudoephedrine showed an inhibitory effect on the replication of IAV. These results give clear evidence that pseudoephedrine is a potential anti-influenza drug by blunting cytokine storms and inhibition of replication of IAV, and following these results, we speculate that it should be tested in the novel coronavirus pneumonia (COVID-19, a severe epidemic in China currently) in which the cytokine storms play a key role to damage bronchi and lung in the early stage.
Humans have been fought with the flu all the time, and up till now. In recent years, the pandemic virus, including avian influenza, infectious atypical pneumonia and the current novel coronavirus COVID-19, are life-threatening respiratory diseases. These diseases have become serious public health problems in worldwide, and in many developing countries, the problem is devastating. It is well known that the development influenza vaccine is hysteretic generally, and some targeted drugs have been developed for treating influenza, such as Oseltamivir, but the effect is lower cost-effectiveness and moved out of the recommended list by WHO [1]. It is necessary to find effective drugs.
Ephedra is be used clinically such a long time in China. The record that Ephedra treated cold and flu, could be found in ‘Treatise on Cold Induced Febrile Disease (shang han lun)’ which is ancient traditional Chinese medical literature. Based on previous reports [2,3], we speculated that ephedra and its main alkaloids, including pseudoephedrine and ephedrine, should have great therapeutic effects in the influenza, and more cost-efficient. Pseudoephedrine and ephedrine have been used as adrenergic agents clinically. However, researches only focused on their sympathomimetic effects, and in fact, they have excellent anti-inflammatory activities [3]. Pseudoephedrine is be approved by FDA for nasal decongestant [4], and it has lower sympathomimetic action than ephedrine. We suggest that pseudoephedrine could be a potential inflammation inhibitor in influenza.
Animals
Ninety-Six (half males and females) specific pathogen free C57BL/6 mice weighing 20 ± 1 g were purchased from Guangdong Medical Laboratory Animal Center. Mice were placed in a controlled environment of (23°C ± 1°C) and (50% ± 5%) relative humidity with free access to food and water for 14 days, under a 12h light/dark cycle. Experiments were performed under the supervision and assessment of the Laboratory Animal Ethics Committee of Jinan University. All experimental procedures abided by the Statute on the Administration of Laboratory Animal approved by China Council 1988.
Grouping, virus and treatment
Groups: Mice were randomly divided into the following four groups, with 24 mice in each group:
Group A: normal control group (normal con);
Group B: IAV control group (V con);
Group C: IAV+ oseltamivir treatment group (V oseltamivir) (Oseltamivir, 195 mg/kg, po);
Group D: IAV + PE treatment group (V PE) (Pseudoephedrine, 30 mg/kg, po). In parallel, mice were randomly divided into the same four groups (each 5 mice) for the survival experiment.
Virus
Virus strain: type A influenza virus, FM1 mouse lung adapted strain (FM1), stored at -80 °C. It was provided by the Department of Microbiology and Immunology, School of Basic Medical Sciences, Jinan University. The hemagglutination titer was 1:40 after two times of routine chick embryo resuscitation. The mortality of mice 14 days after virus infection at different concentrations was determined by double dilution method. The virus concentration causing 20% mouse mortality (blood coagulation titer 1:640) was used and 50 μL of virus solution was given to each mouse.
Compound
Pseudoephedrine was purchased from the Guangzhou Institute for the Control of Pharmaceutical and Biological Products (China) and purities of Pseudoephedrine is > 98%. Oseltamivir Phosphate Capsules was obtained from Yichang Yangtze River East Sunshine pharmaceutical Ltd. (China) and purities is > 98%.
Treatment
The dose of oseltamivir is based on previous research [5], and pseudoephedrine is based on our pre-experiment. The mice in Group B, Group C and Group D were fully anesthetized by inhalation of diethyl ether and then infected by intranasal application of 20% LD50 FM1 influenza virus suspension on day 1. This procedure leads to upper and lower respiratory tract infection. And mice in Group A were applied saline after anesthetized by inhalation of diethyl ether. Drug-treatment was started on day 2 (Group C and Group D). PE or oseltamivir was dissolved in physiological saline and administered by intraperitoneal injection for 4 days after influenza virus infection. For Group B, each mouse was administered by intraperitoneal injection with 1ml saline per day. Every eight mice in each group were euthanized on day 2, day 4 and day 6 respectively.
Survival experiment
Mice were intranasally challenged with a diluted virus (10 × LD50) 2 h before oral administration. Normal control mice were intranasally challenged with equal volumes of virus diluents. The mice were then orally administered once a day for 7 days. All groups were monitored for 14 days after infection. Body weights and numbers of mice were recorded daily. The Life span and the survival rate were calculated. The changes in body weight were displayed as the percentage of the mouse’s weight on the day before viral infection. In addition, clinical signs including ruffled fur, redness around the eyes, nose or mouth, hunched back, altered respiration and unresponsiveness were observed.
Examination of histopathology
The lung samples were obtained. 0.5 cm of lung tissue were removed for analysis, fixed with 4% paraformaldehyde, and then modified and trimmed by a blade. The appropriate lung part was selected and treated with the following steps: rinsing, dehydration, treatment by a transparent agent, immersing and other steps. After the sections were treated with drying, dewaxing, hydration and other steps, and stained with hematoxylin and eosin. The tissues were sectioned, and central tissue organization observed. The size of the microscopic field employed in the analysis was 200X.
RT-qPCR of TLR7, MyD88, MAVS, RIG-I and NF-kappa B p65 mRNA and relative expression of the influenza A virus in lung
The lung samples were obtained. mRNA expression of TLR7, MyD88, MAVS, RIG-I and NF-kappaB was measured using quantitative real-time reverse transcriptase PCR (RT-qPCR), as well as IAV replication in lung. Total RNA was extracted by RNAiso Plus (Cat. #9108, TaKaRa, Japan) according to the manufacturer’s instructions. cDNA synthesis and real-time PCRs were carried out using the CFX Connect Real-Time PCR Detection system (BIO-RAD, USA) with PrimeScript RT reagent kits (RR047A, TaKaRa) and SYBR Premix EX Taq II (RR820A, TaKaRa) according to the manufacturer’ s instructions. Primers were synthesized by Generay Biotech Co. (Shanghai, China). All primers for RT-qPCR are presented in table 1. After RT-qPCR, analysis of the relative gene expression levels was performed using the 2−ΔΔCT method. Each sample was measured three times and averaged. Gene expression in the V oseltamivir and V PE was expressed relative to the V con.
| Table 1: Primers used for RT-qPCR analysis. | ||
| Gene | Primer | Sequence |
| GAPDH | Forward primer | 5'-TGATGACATCAAGAAGGTGGTGAAG-3' |
| Reverse primer | 5'-TCCTTGGAGGCCATGTAGGCCAT-3' | |
| TLR7 | Forward primer | 5'-GGGTCCAAAGCCAATGTG-3' |
| Reverse primer | 5'-TGTTAGATTCTCCTTCGTGATG-3' | |
| MyD88 | Forward primer | 5'-CGATTATCTACAGAGCAAGGAATG-3' |
| Reverse primer | 5'-ATAGTGATGAACCGCAGGATAC-3' | |
| MAVS | Forward primer | 5'- AGAGCATCAAGAGCAAGAAC-3' |
| Reverse primer | 5'- GGAGACACAGGTCCATAGG-3' | |
| RIG-I | Forward primer | 5'- CAGAGCCAGCGGAGATAAC-3' |
| Reverse primer | 5'-GGTCAGGAGGAAGCACTTG-3' | |
| NF-kappaB p65 | Forward primer | 5'-ATTCTGACCTTGCCTATCTAC-3' |
| Reverse primer | 5'-TCCAGTCTCCGAGTGAAG-3' | |
| Virus Replication | Forward primer | 5’-GACCAATCCTGTCACCTCTGAC-3’ |
| Reverse primer | 5’-GGGCATTTGGACAAACGTCTACG-3’ | |
| RT-qPCR, quantitative real-time polymerase chain reaction. | ||
Cytokine measurements
Determination of the level of cytokines by ELISA. The amount of cytokine in serum and lung tissue was determined by Mouse TNF alpha or Mouse IFN gamma ELISA Ready-SET-Go! 2 x 96 tests (Cat. #8588732422 and Cat. #8588731422, ebioscience, The USA) according to manufacturer’s instructions. Briefly: Cytokines were captured from serum and lung tissue via particular mouse cytokine-specific immuno-capture antibody bound to the solid phase of 96-well micro-titration plate. Examined cytokines were detected after overnight incubation at 40 oC. The biotin-conjugated detector antibody specific for each cytokine was added to the reaction mixture and subsequently incubated with horseradish peroxidase-conjugated avidin. Then, after a period of 30 min, the substrate tetramethylbenzidine with hydrogen peroxide was added. The reaction was stopped with 1 M H2SO4 and the color reaction was evaluated by measuring the absorbance at 450 and 570 nm on ELISA reader Epoch 2 (BioTek Instruments, Inc., Vermont, and The USA). The concentrations of cytokines in the particular samples (pg/100μl) were calculated from the calibration curve according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were carried out using SPSS 22 for Macintosh (IBM software, NY, USA). All data are presented as mean±SD. Groups were compared using one-way ANOVA, followed by post-hoc Student-Newman-Keuls tests. The values of protein band density obtained from gel analysis and band densitometry were calculated. These values were expressed as TLR7, NF-kappaB or MyD88/GAPDH ratio for each sample. The averages for different groups were compared using ANOVA followed by the Newman–Keuls test. A p value of < 0.01 was considered to be statistically significant.
Pseudoephedrine can significantly improve the survival rate and body weight of H1N1 virus-infected mice
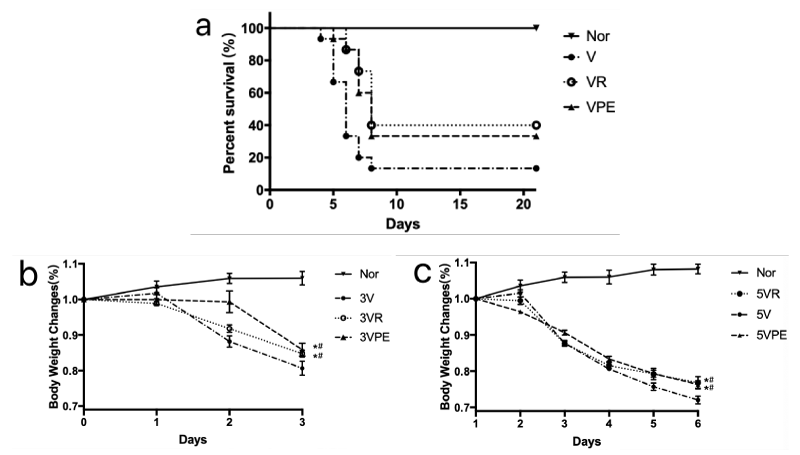
In the survival experiment, the IAV-infected mice presented clinical signs such as a tendency to huddle, piloerection, lethargy, ruffled fur, weight loss, respiratory distress and lack of appetite on the third day. Mice in PE group have relatively more activity but no irritability or convulsions. There was no death in the normal group during 21d observation. Mice began to die from day 4 after infection in model group (1 deaths out of 15 mice). The IAV-infected induced the 86.6% lethality rate in 8d. PE or oseltamivir treated mice were obviously protected from died (lethality rate: Oseltamivir, 40%; PE 33.33%). PE significantly increased survival rate as well as Oseltamivir. Meanwhile, the life span was significantly increased in PE and oseltamivir treated groups when compared with model group. Compared with model group, PE significantly prolonged the life span of IAV-infected mice. Mice from model group exhibited an obvious weight loss from 3rd day. PE and oseltamivir effectively protected the infected mice from weight loss caused by IAV infection (Figure 1).
Figure 1: Survival rate and body weight change after treatment with PE or oseltamivir in infected mice. Group V, VR and VPE were fully anesthetized by inhalation of diethyl ether and then infected by intranasal application of 20% LD50 FM1 influenza virus suspension on day 1, Nor was saline in the same way. The next day, treatments for each group were started with either oseltamivir, pseudoephedrine or saline. In the A, observed for 20 days for 15 mice of each group, and in the B and C, the animals of 7 mice of each group were sacrificed at 1, 3, 5 days, withdrawing serum and lung respectively. #p < 0.05 compared to Nor, *p < 0.05 compared to V.
Pseudoephedrine blunts cytokine storms and their inflammatory gene expression
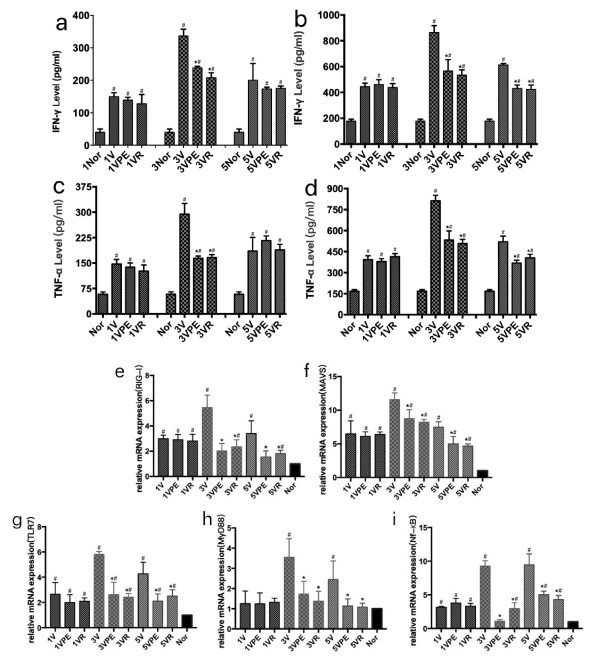
In the early stage of influenza, a cytokine storm was formed. The release of a large number of cytokines is an important factor in the damage of cells and tissue [6]. In our experiments, pseudoephedrine significantly reduced the release of cytokines. IFN-γ and TNF-α in lung tissue decreased significantly on the third day (Figure 2A-B), and decreased significantly on the third and fifth days in serum (Figure 2C-D).
Figure 2: Changes in cytokines in lung tissue and serum and expression of related genes. The model and treatment are the same as figure1. #p < 0.05 compared to Nor, *p < 0.05 compared to V.
The RIG-I and MAVS genes of lung tissue after influenza virus infection were highly expressed on day 3 and day 5, while pseudoephedrine significantly inhibited the expression of RIG-1 and MAVS genes (Figure 2E-F). In another inflammatory pathway, pseudoephedrine also showed excellent inhibition of gene expression of TRL7, MyD88 and NF-κB (Figure 2G-I) [7].
Pseudoephedrine can significantly inhibit the replication of IAV
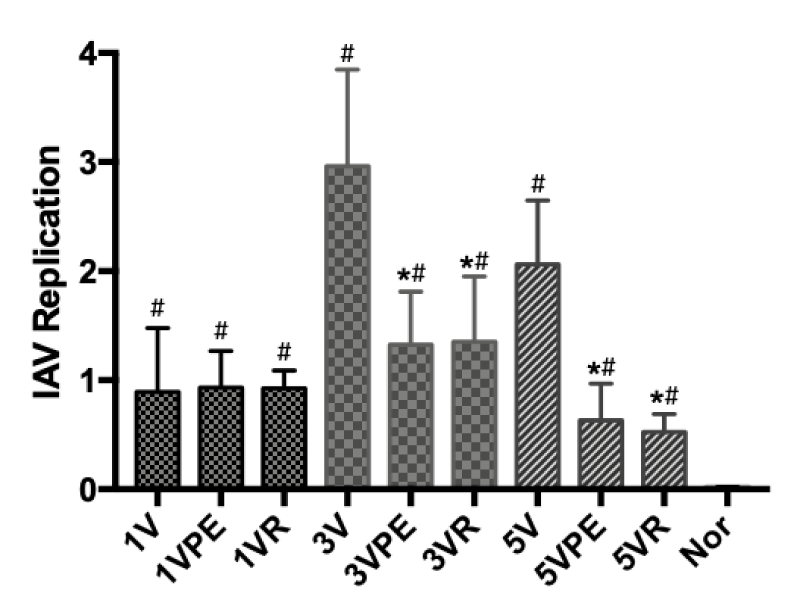
After the virus enters the upper respiratory tract, the geometrical replication of the virus causes epithelial cell lesions to be the main mechanism of influenza pathogenesis. In this experiment, pseudoephedrine significantly inhibited the replication of AIV virus at 3 and 5 days (Figure 3).
Figure 3: Virus replication. The model and treatment are the same as Figure 1. #p < 0.05 compared to Nor, *p < 0.05 compared to V.
Pseudoephedrine can significantly improve lung pathological damage after IAV infection
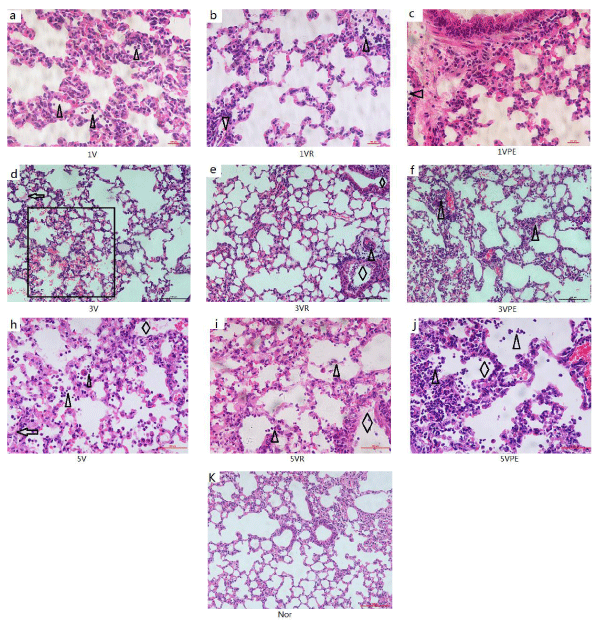
IVA-infected pneumonia is characterized by pulmonary congestion, edema, bloody fluid in the trachea and bronchi, mucosa hyperemic, with loss of normal ciliated epithelial cells, Submucosal hyperemia and focal hemorrhage, edema and inflammatory cellular infiltration. The alveolar spaces contain various numbers of neutrophils and mononuclear cells admixed with fibrin. The alveoli of the lower lungs often have intra-alveolar hemorrhage and acellular, hyaline membranes [8]. In this experiment, all groups had no abnormalities in the tracheal wall at different time points (no shown), the lesions concentrated in the bronchioles, alveoli and interstitium. On the day 1, it showed inflammatory cellular infiltration in the small pulmonary vessels. On the day 3, the model group showed intra-alveolar hemorrhage and hyaline membranes, bronchiole wall necrosis, a large number of inflammatory cells infiltration (Lymphocytes, macrophages and neutrophils). On the day 5, bronchiole wall necrosis was more and more obvious. Compared with the model, the group of VR and VPE have a significant improvement for the overall lesions (Figure 4).
Figure 4: H&E staining of morphological section of the lung the model and treatment are the same as figure 1. (k) Normal lung, bronchus and alveoli. In (a),(b),(c) for 1 day, significant inflammatory cellular infiltration in the small pulmonary vessels (triangles).In (d),(e),(f) for 3 days, and (h)(i)(j) for 5 day, intra-alveolar hemorrhage(rectangle) and hyaline membranes(arrows), bronchiole wall necrosis(rhombus), intra-alveolar infiltration(triangles).(a-c) and (h-j) are400X, the others are 200X.
Our previous researches have shown that ephedra and its major alkaloids have excellent anti-inflammatory effects and are highly protective in D-galactosamine/lipopolysaccharide-induced acute liver failure in rats, inhibiting cytokines such as TNF-α [3,9].
In the early stage of IVA infection, RIG-I (retinoic acid-inducible gene I) was conformational change, and activating MAVS (mitochondrial antiviral signaling protein) between their CARD (caspase recruitment domain) and triggering an antiviral response including IRF-3, IRF-7, NF-κB pathway to release abundant cytokines [10]. Cytokine storm is the cause of systemic inflammatory response syndrome or multi organs failure [6]. In the present study, IFN-γ and TNF-α were significantly elevated in the early stage of IVA infection. The RIG-1 and MAVS genes were also highly expressed. Pseudoephedrine significantly inhibited the expression of IFN-γ, TNF-α, RIG-1 and MAVS. In the early stage of inflammatory response to viral infection, TRL7 and MyD88 are important pathways for cytokine amplification [7], Pseudoephedrine showed a good inhibitory effect in this study.
Transcription and replication of IVA is a complex process that takes place within the nucleus. The nucleoprotein and matrix protein M1 play an important role in the replication process, but now there are fewer medicine that actually inhibit viral replication [11,12]. Oseltamivir inhibits neuraminidase which cleaves the terminal a-Neu5Ac residues from the newly synthesized virion progeny and let it elute from the infected cell and seek new host cells to infect and stops IVA spreading [13]. In this experiment, pseudoephedrine has a very significant inhibitory effect on IVA RNA replication, which surprised us. The inhibition mechanism needs further study. Because of the inhibiting effect of pseudoephedrine on cytokine storm and virus replication, the pathological manifestations of IVA infection in the lungs are also significantly improved, specifically keep the relative integrity of the endothelium of Bronchioles and alveolar.
Ephedra is a drug that has been used by humans for thousand years. From its symptom manifestation of application, this disease is very similar to the performance of the flu. Ephedrine and pseudoephedrine are the main alkaloids of Ephedra which has a perfect structure to produce all kinds of narcotics including ecstasy, amphetamin chloride, but they have no addiction actually. They are innocent. Although ephedrine has a strong sympathomimetic effect, pseudoephedrine has a moderate effect and even has a certain antihypertensive effect after tolerance [14].
Due to the short half-life of pseudoephedrine and the short duration of action, we recommend the use of sustained release agents of pseudoephedrine for clinical treatment. Even if the fever has gone down, it should be taken three times within 24 hours. In China, a sustained release dosage called CONTACNT (product of GSK) contains 90 mg pseudoephedrine hydrochloride and 4 mg chlorphenamine maleate, so it is also used in the allergic dermatosis and common cold and flu.
This study showed that pseudoephedrine could protect mice from infection of H1N1 virus. Pseudoephedrine exerts an excellent anti-influenza effect by inhibiting related inflammatory genes and pacifying cytokines such as IFN-γ, TNF-α, etc. and inhibiting the replication of IVA. In addition to serving as a nasal decongestant, ephedrine has a slight adrenal effect and the role of inhibition of vascular exudation [15], which may also have a positive effect on early exudation of influenza. We suggest that pseudoephedrine is a precious gift from nature that is worthy for further research and exploration. Pseudoephedrine is cheap, easy to get, and effective drug.
The novel coronavirus pneumonia, COVID-19, is a current severe infectious disease in China. Critical patients showed a high fatality rate and inflammatory cytokine storm is the main character in severe cases and deaths [16]. We suggest here that pseudoephedrine (including Ephedra and ephedrine also) might have a blocking effect on cytokine storm, alleviating development of the disease.
We thank Tin Ka Ping Center for Medical Experiments (Jinan University) for providing experimental place. This work was supported by the National Natural Science Foundation of China (No. 81830114 and No. 81774164), Natural Science Foundation of Guangdong, China (No. 2017A030313737) and 2017gxbjZD45, SHUTCM (A1-N1920501020710).
- Kmietowicz Z. WHO downgrades oseltamivir on drugs list after reviewing evidence. BMJ. 2017; 357: j2841. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28607038
- Wang C, Cao B, Liu QQ, Zou ZQ, Liang ZA, et al. Oseltamivir compared with the Chinese raditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann Intern Med. 2011; 155: 217-225. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21844547
- Wu Z, Kong X, Zhang T, Ye J, Fang Z, et al. Pseudoephedrine/ephedrine shows potent anti-inflammatory activity against TNF-α-mediated acute liver failure induced by lipopolysaccharide/d-galactosamine. Eur J Pharmacol. 2014; 724: 112-121. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24365491
- Gelotte CK, Albrecht HH, Hynson J, Gallagher V. A Multicenter, Randomized, Placebo-Controlled Study of Pseudoephedrine for the Temporary Relief of Nasal Congestion in Children with the Common Cold. J Clin Pharmacol. 2019; 59: 1573-1583. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31274197
- Marathe BM, Mostafa HH, Vogel P, Pascua PNQ, Jones JC, et al. A pharmacologically immunosuppressed mouse model for assessing influenza B virus pathogenicity and oseltamivir treatment. Antiviral Res. 2017; 148: 20-31. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29100887
- Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014; 124: 188-195. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24876563
- Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc Natl Acad Sci U S A. 2014; 111: 3799-3804. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24572573
- Richman DD, Whitley RJ, Hayden FG. Clinical Virology. Third Edition ed. 2009.
- Han Y, Zhu J, Wu Z. Ephedra protects rats against acute liver failure induced by D-galactosamine and lipopolysaccharide. Zhonghua Gan Zang Bing Za Zhi. 2016; 24: 127-129. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26983481
- Jacobs JL, Coyne CB. Mechanisms of MAVS regulation at the mitochondrial membrane. J Mol Biol. 2013; 425: 5009-5019. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24120683
- Lee WS, Hsu CY, Wang PL, Huang CY, Chang CH, et al. Identification and characterization of the nuclear import and export signals of the mammalian Ste20-like protein kinase 3. FEBS Lett. 2004; 572: 0-45. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15304321
- Cao S, Liu X, Yu M, Li J, Jia X, et al. A nuclear export signal in the matrix protein of Influenza A virus is required for efficient virus replication. J Virol. 2012; 86: 4883-4891. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22345442
- Feng E, Ye D, Li J, Zhang D, Wang J, et al. Recent advances in neuraminidase inhibitor development as anti-influenza drugs. Chem Med Chem. 2012; 7: 1527-1536. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22807317
- Dickerson J, Perrier D, Mayersohn M, Bressler R. Dose tolerance and pharmacokinetic studies of L (+) pseudoephedrine capsules in man. Eur J Clin Pharmacol. 1978; 14: 253-259. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/729619
- Rang H, Henderson G, Flower R, Ritter J. Rang & Dale's Pharmacology. Elsevier Health Sciences. 2007.
- Tetro JA. Is COVID-19 Receiving ADE from Other Coronaviruses? Microbes Infect. 2020.